Enter your answer in the provided box. The equilibrium constant K, for the equation 2H2(g) + CO(g) = CH3OH(g) is 13 at a certain temperature. If there are 2.21 x 10-2 moles of H, and 2.33 x 103 equilibrium in a 4.31-L flask, what is the concentration of CO? moles of CH3OH at M
Enter your answer in the provided box. The equilibrium constant K, for the equation 2H2(g) + CO(g) = CH3OH(g) is 13 at a certain temperature. If there are 2.21 x 10-2 moles of H, and 2.33 x 103 equilibrium in a 4.31-L flask, what is the concentration of CO? moles of CH3OH at M
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 62QRT
Related questions
Question
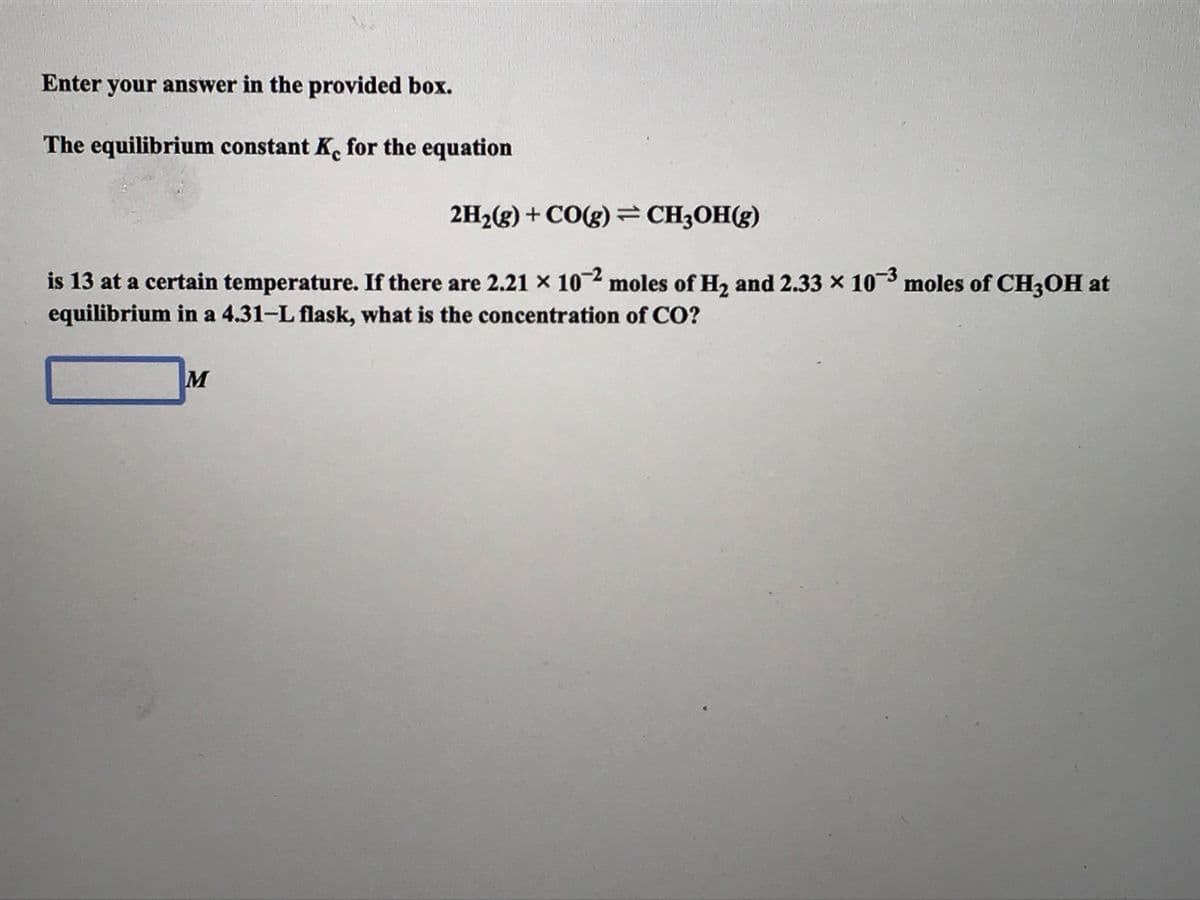
Transcribed Image Text:Enter your answer in the provided box.
The equilibrium constant K. for the equation
2H2(g) + CO(g)=CH3OH(g)
is 13 at a certain temperature. If there are 2.21 × 102 moles of H2 and 2.33 x 103 moles of CH;OH at
equilibrium in a 4.31-L flask, what is the concentration of CO?
M
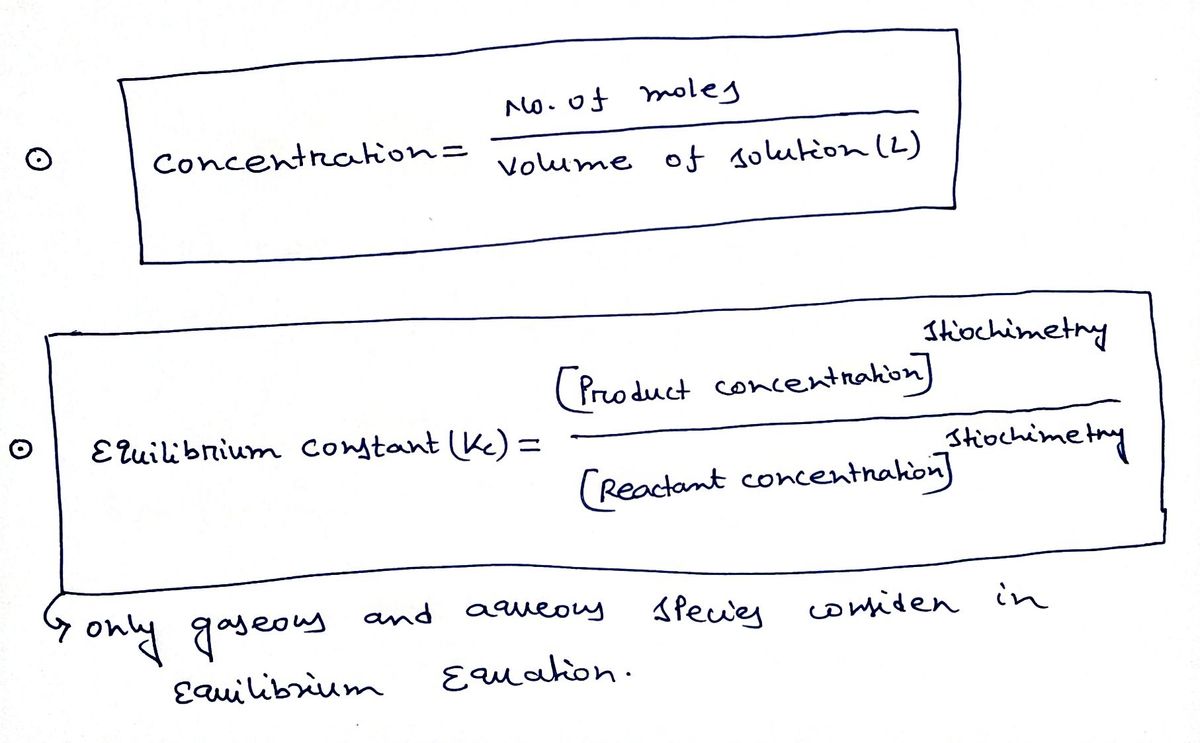
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning