* Enthalpy Constants Enthalpy H is a measure of the energy content of a system at constant pressure. Chemical reactions involve changes in enthalpy, AH, which can be measured and calculated: Part A AH =Eprodncte MAH° - Enctants RAH %3D Determine the enthalpy for this reaction: where the subscript "rxn" is for "enthalpy of reaction" and "f" is for "enthalpy of formation" and m and n represent the appropriate stoichiometric coefficients for Al(OH)3 (s) + 3HCI(g)→AICl3 (s) + 3H;O(1) Express your answer in kilojoules per mole to one decimal place. each substance. > View Available Hint(s) The following table lists some enthalpy of formation values for selected substances. AH (kJ/mol) ? Substance HCl(g) -92.0 Al(OH)3(s) AH = kJ/mol -1277.0 H20(1) -285.8 AlCk (s) -705.6 Submit Previous Answers H20(g) -241.8 X Incorrect; Try Again; 26 attempts remaining
* Enthalpy Constants Enthalpy H is a measure of the energy content of a system at constant pressure. Chemical reactions involve changes in enthalpy, AH, which can be measured and calculated: Part A AH =Eprodncte MAH° - Enctants RAH %3D Determine the enthalpy for this reaction: where the subscript "rxn" is for "enthalpy of reaction" and "f" is for "enthalpy of formation" and m and n represent the appropriate stoichiometric coefficients for Al(OH)3 (s) + 3HCI(g)→AICl3 (s) + 3H;O(1) Express your answer in kilojoules per mole to one decimal place. each substance. > View Available Hint(s) The following table lists some enthalpy of formation values for selected substances. AH (kJ/mol) ? Substance HCl(g) -92.0 Al(OH)3(s) AH = kJ/mol -1277.0 H20(1) -285.8 AlCk (s) -705.6 Submit Previous Answers H20(g) -241.8 X Incorrect; Try Again; 26 attempts remaining
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 12QAP: The heat of neutralization, Hneut, can be defined as the amount of heat released (or absorbed), q,...
Related questions
Question
100%
Transcribed Image Text:36 of 42
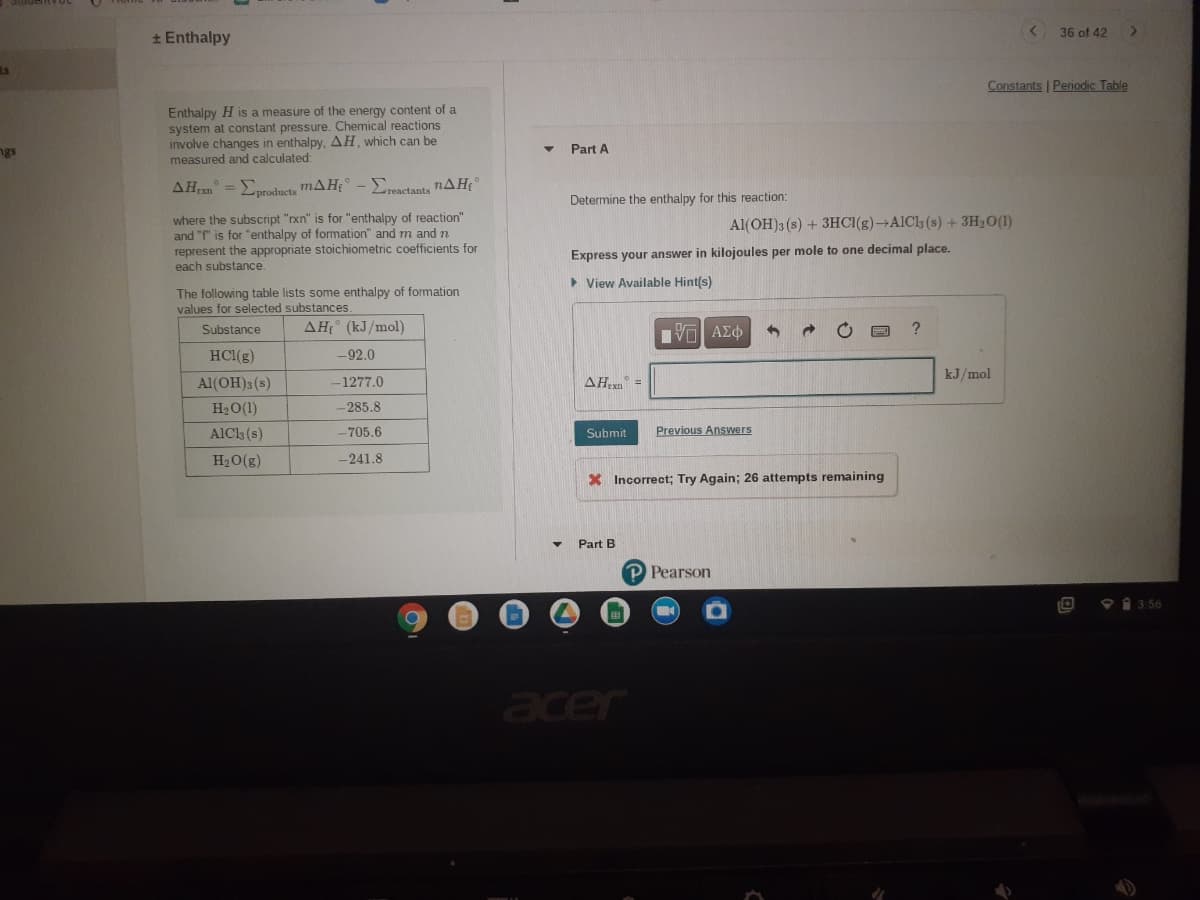
* Enthalpy
Constants Periodic Table
Enthalpy H is a measure of the energy content of a
system at constant pressure. Chemical reactions
involve changes in enthalpy, AH, which can be
measured and calculated:
ngs
Part A
AHan =Eproducts mAH;
Eresctants nAH
Determine the enthalpy for this reaction:
where the subscript "rxn" is for "enthalpy of reaction"
and "f" is for "enthalpy of formation" and m and n
represent the appropriate stoichiometric coefficients for
each substance.
Al(OH)3 (s) + 3HC1(g)→AIC13 (s) + 3H20(1)
Express your answer in kilojoules per mole to one decimal place.
> View Available Hint(s)
The following table lists some enthalpy of formation
values for selected substances
Substance
AH (kJ/mol)
HCl(g)
-92.0
kJ/mol
Al(OH)3 (s)
-1277.0
AH =
H20(1)
-285.8
AlCk (s)
-705.6
Submit
Previous Answers
H20(g)
-241.8
X Incorrect; Try Again; 26 attempts remaining
Part B
P Pearson
1 3.56
acer
Transcribed Image Text:dent.
Elk Grove Unified-
O My Profile- Zoom
Results of the chec.
My Citation list 9/2.
Pearson Sign In
O College Board - SAT..
>>
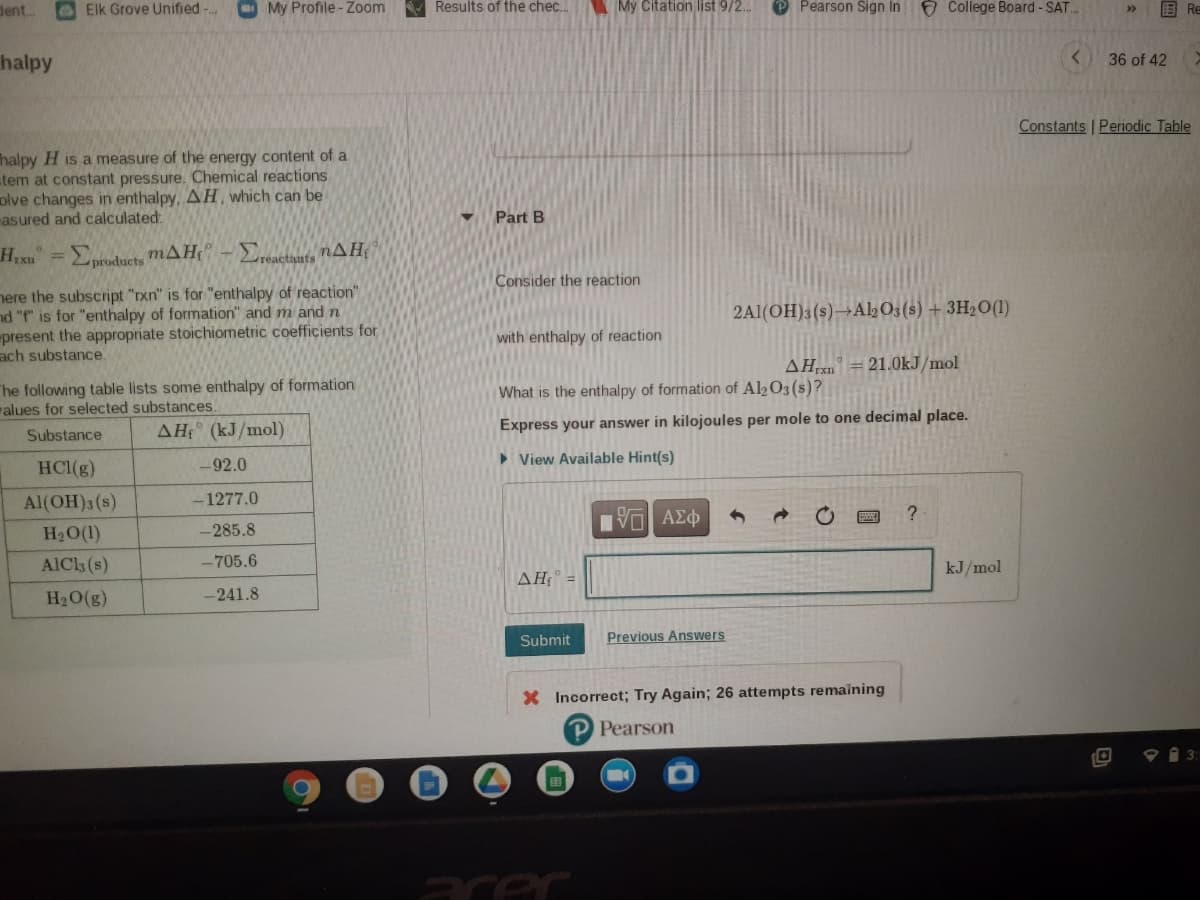
halpy
36 of 42
Constants Periodic Table
halpy H is a measure of the energy content of a
tem at constant pressure. Chemical reactions
olve changes in enthalpy, AH, which can be
asured and calculated:
Part B
Hixu =
Eproducts mAH" -
bactauts
Consider the reaction
nere the subscript "rxn" is for "enthalpy of reaction"
nd "f" is for "enthalpy of formation" and m and n
present the appropriate stoichiometric coefficients for
ach substance.
2A1(OH)3(s)→AL03(s) + 3H2O(1)
with enthalpy of reaction
AH = 21.0kJ/mol
The following table lists some enthalpy of formation
alues for selected substances.
What is the enthalpy of formation of Al½O3(s)?
AH (kJ/mol)
Express your answer in kilojoules per mole to one decimal place.
Substance
HCl(g)
-92.0
> View Available Hint(s)
Al(OH)3 (s)
-1277.0
H2O(1)
-285.8
AICls (s)
-705.6
kJ/mol
AH =
H2O(g)
-241.8
Submit
Previous Answers
X Incorrect; Try Again; 26 attempts remaining
P Pearson
O i 3:
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning