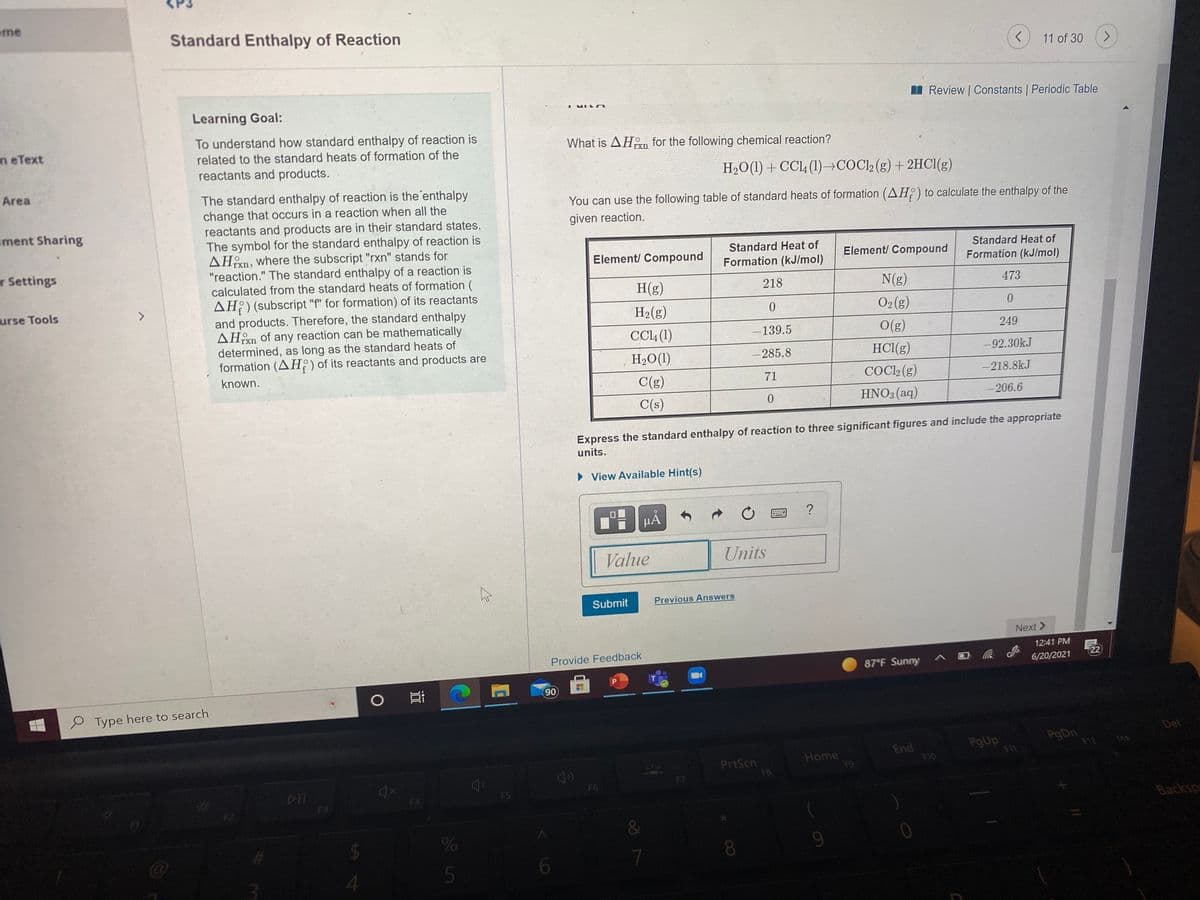
What is AHan for the following chemical reaction? H20(1) + CC4 (1)-→COCl2(g) + 2HC1(g) You can use the fllowing table of standard heats of formation (AH) to calculate the enthalpy of the given reaction. Standard Heat of Formation (kJ/mol) Standard Heat of Element/ Compound Element/ Compound Formation (kJ/mol) H(g) 218 N(g) 473 H2(g) O2(g) 249 CL (1) H2O(1) 139.5 O(g) 285.8 HCI(g) -92.30kJ COC (g) -218.8kJ C(g) 71 C(s) HNO:(aq) 206.6 Express the standard enthalpy of reaction to three significant figures and include the appropriate units. • View Available Hint(s) HA Value Units Submit Previous Answers
What is AHan for the following chemical reaction? H20(1) + CC4 (1)-→COCl2(g) + 2HC1(g) You can use the fllowing table of standard heats of formation (AH) to calculate the enthalpy of the given reaction. Standard Heat of Formation (kJ/mol) Standard Heat of Element/ Compound Element/ Compound Formation (kJ/mol) H(g) 218 N(g) 473 H2(g) O2(g) 249 CL (1) H2O(1) 139.5 O(g) 285.8 HCI(g) -92.30kJ COC (g) -218.8kJ C(g) 71 C(s) HNO:(aq) 206.6 Express the standard enthalpy of reaction to three significant figures and include the appropriate units. • View Available Hint(s) HA Value Units Submit Previous Answers
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter15: Energy And Chemical Change
Section: Chapter Questions
Problem 112A: sample of natural gas is analyzed and found to be88.4% methane (CH4) and 11.6% ethane (C2H6) bymass....
Related questions
Question
Transcribed Image Text:eme
Standard Enthalpy of Reaction
11 of 30
<.
Learning Goal:
I Review | Constants | Periodic Table
। ५
To understand how standard enthalpy of reaction is
n eText
What is AHn for the following chemical reaction?
related to the standard heats of formation of the
reactants and products.
H2O(1) + CCL (1)→COC2 (g) + 2HCI(g)
Area
The standard enthalpy of reaction is the'enthalpy
change that occurs in a reaction when all the
reactants and products are in their standard states.
The symbol for the standard enthalpy of reaction is
AHn, where the subscript "rxn" stands for
"reaction." The standard enthalpy of a reaction is
calculated from the standard heats of formation (
AH?) (subscript "f" for formation) of its reactants
You can use the following table of standard heats of formation (AH:) to calculate the enthalpy of the
ument Sharing
given reaction.
Standard Heat of
Standard Heat of
T Settings
Element/ Compound
Formation (kJ/mol)
Element/ Compound
Formation (kJ/mol)
H(g)
218
N(g)
473
urse Tools
O2(g)
and products. Therefore, the standard enthalpy
AHn of any reaction can be mathematically
determined, as long as the standard heats of
formation (AH;)of its reactants and products are
H2(g)
CCL4 (1)
O(g)
139.5
249
H2O(1)
-285.8
HCI(g)
-92.30kJ
known.
C(g)
71
COCI2 (g)
- 218.8kJ
C(s)
HNO3(aq)
-206.6
Express the standard enthalpy of reaction to three significant figures and include the appropriate
units.
• View Available Hint(s)
?
HA
Value
Units
Submit
Previous Answers
Next >
Provide Feedback
12:41 PM
22
87°F Sunny
6/20/2021
T
90
P Type here to search
Del
End
F10
PgUp
F11
PgDn
F12
Ins
PrtScn
F8
Home
F9
F7
F6
F5
F4
F2
F3
4
5
6
6,
88
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT