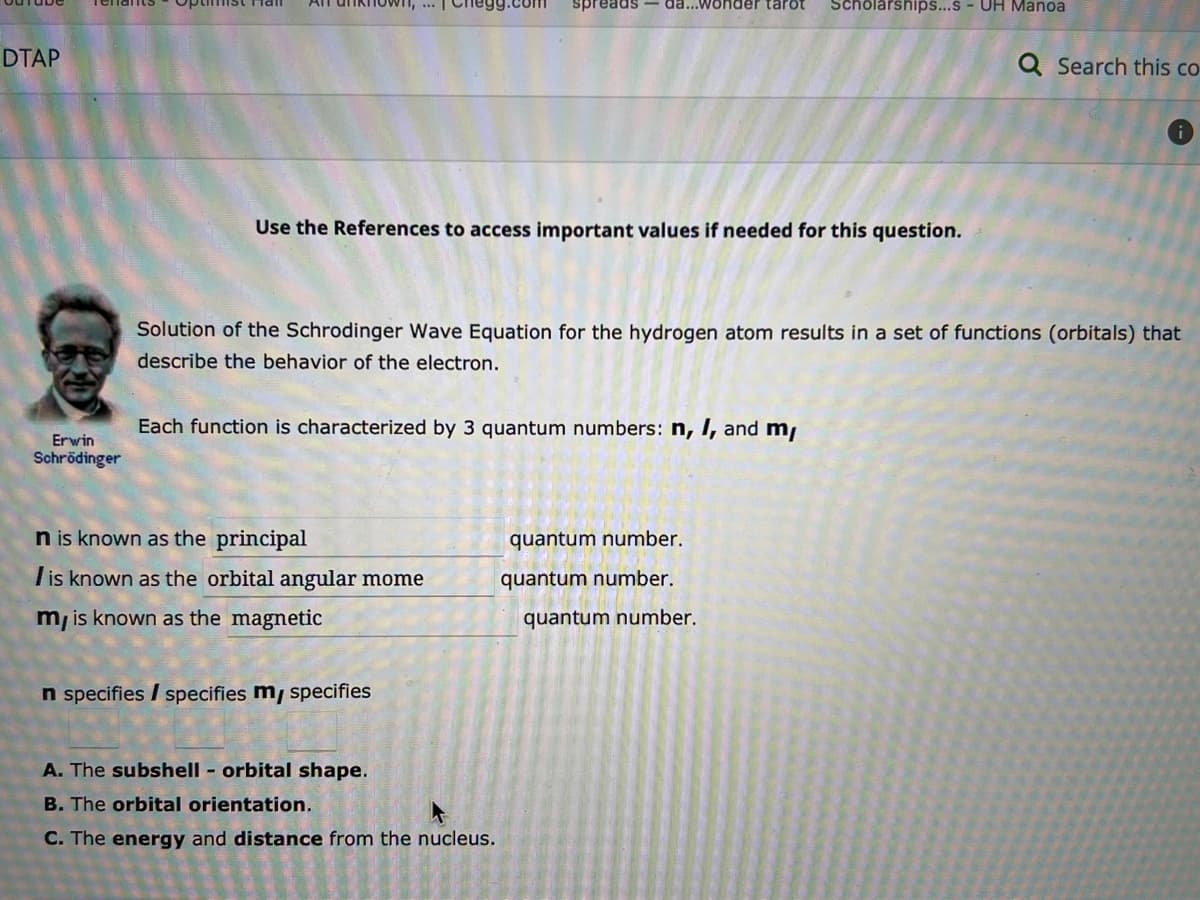
Erwin Schrödinger Use the References to access important values if needed for this question. Solution of the Schrodinger Wave Equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is characterized by 3 quantum numbers: n, I, and m/ n is known as the principal is known as the orbital angular mome m, is known as the magnetic n specifies / specifies my specifies A. The subshell - orbital shape. B. The orbital orientation. C. The energy and distance from the nucleus. quantum number. quantum number. quantum number.
Erwin Schrödinger Use the References to access important values if needed for this question. Solution of the Schrodinger Wave Equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is characterized by 3 quantum numbers: n, I, and m/ n is known as the principal is known as the orbital angular mome m, is known as the magnetic n specifies / specifies my specifies A. The subshell - orbital shape. B. The orbital orientation. C. The energy and distance from the nucleus. quantum number. quantum number. quantum number.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 45AP: Suppose an atom in an excited state can return to the ground state in two steps. It first falls to...
Related questions
Question
100%
I kind of filled out the second one I’m not entirely sure if it’s right but if any part of it is wrong can you please show and explain the correct answer.
Transcribed Image Text:DTAP
Erwin
Schrödinger
Use the References to access important values if needed for this question.
n is known as the principal
I is known as the orbital angular mome
m, is known as the magnetic
spreads-da...wonder tarot Scholarships...s - UH Manoa
Solution of the Schrodinger Wave Equation for the hydrogen atom results in a set of functions (orbitals) that
describe the behavior of the electron.
Each function is characterized by 3 quantum numbers: n, I, and m/
n specifies / specifies my specifies
A. The subshell - orbital shape.
B. The orbital orientation.
C. The energy and distance from the nucleus.
quantum number.
quantum number.
Q Search this co
quantum number.
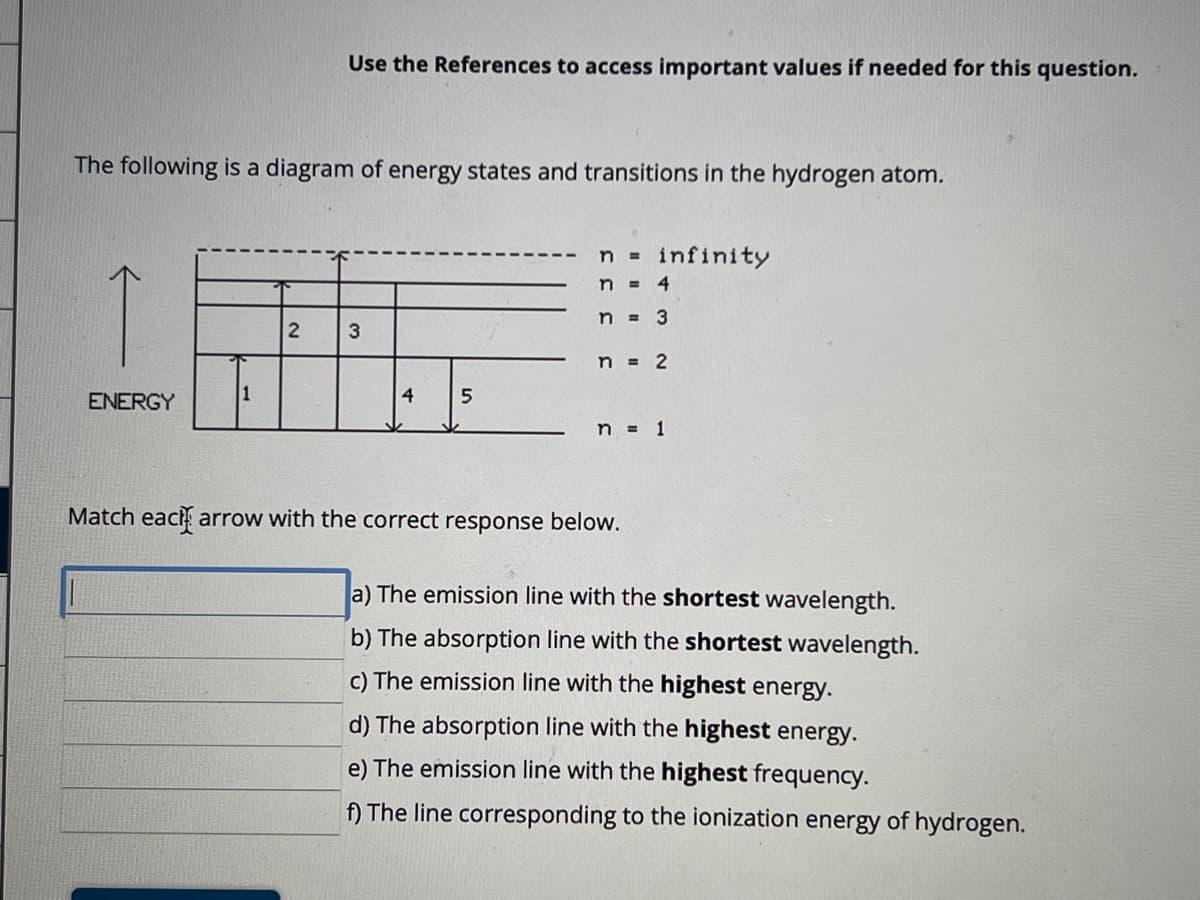
Transcribed Image Text:The following is a diagram of energy states and transitions in the hydrogen atom.
ENERGY
1
Use the References to access important values if needed for this question.
2
3
4
5
n = infinity
n = 4
n = 3
n = 2
n = 1
Match each arrow with the correct response below.
a) The emission line with the shortest wavelength.
b) The absorption line with the shortest wavelength.
c) The emission line with the highest energy.
d) The absorption line with the highest energy.
e) The emission line with the highest frequency.
f) The line corresponding to the ionization energy of hydrogen.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
