Estimate the energy difference between AU° and AH° (that is: AU° – ▲H°), in kJ mol−, for the following reaction at 298 K: a. -10.1 kJ mol-1 b. -7.74 kJ mol-1 c. +2.48 kJ mol-¹ d. +6.41 kJ mol-¹ e. +13.3 kJ mol−¹ 2 NaOH(s) CO2(g) →>> Na2CO3(aq) + H2O(l)
Estimate the energy difference between AU° and AH° (that is: AU° – ▲H°), in kJ mol−, for the following reaction at 298 K: a. -10.1 kJ mol-1 b. -7.74 kJ mol-1 c. +2.48 kJ mol-¹ d. +6.41 kJ mol-¹ e. +13.3 kJ mol−¹ 2 NaOH(s) CO2(g) →>> Na2CO3(aq) + H2O(l)
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter17: Conjugation And Molecular Orbital (mo) Theory
Section: Chapter Questions
Problem 8CTQ
Related questions
Question
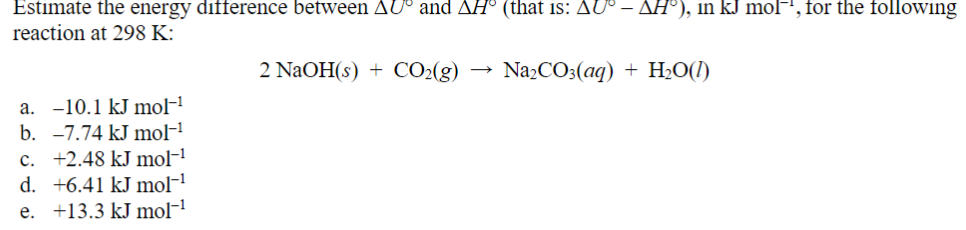
Transcribed Image Text:Estimate the energy difference between AU° and AH° (that is: AU° – ▲H°), in kJ mol−, for the following
reaction at 298 K:
a. -10.1 kJ mol-1
b. -7.74 kJ mol-1
c. +2.48 kJ mol-¹
d. +6.41 kJ mol-¹
e. +13.3 kJ mol−¹
2 NaOH(s) CO2(g) →>> Na2CO3(aq) + H2O(l)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning