Chemistry 9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Steven S. Zumdahl
1 Chemical Foundations 2 Atoms, Molecules, And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding: General Concepts 9 Covalent Bonding: Orbitals 10 Liquids And Solids 11 Properties Of Solutions 12 Chemical Kinetics 13 Chemical Equilibrium 14 Acids And Bases 15 Acid-base Equilibria 16 Solubility And Complex Ion Equilibria 17 Spontaneity, Entropy, And Free Energy 18 Electrochemistry 19 The Nucleus: A Chemist's View 20 The Representative Elements 21 Transition Metals And Coordination Chemistry 22 Organic And Biological Molecules Chapter17: Spontaneity, Entropy, And Free Energy
Chapter Questions Section: Chapter Questions
Problem 1RQ: Define the following: a. spontaneous process b. entropy c. positional probability d. system e.... Problem 2RQ: What is the second law of thermodynamics? For any process, there are four possible sign combinations... Problem 3RQ: What determines Ssurr for a process? To calculate Ssurr at constant pressure and temperature, we use... Problem 4RQ: The free energy change, G, for a process at constant temperature and pressure is related to Suniv... Problem 5RQ: What is the third law of thermodynamics? What are standard entropy values, S, and how are these S... Problem 6RQ: What is the standard free energy change, G, for a reaction? What is the standard free energy of... Problem 7RQ: If you calculate a value for G for a reaction using the values of Gf in Appendix 4 and get a... Problem 8RQ: Consider the equation G = G + RT ln(Q). What is the value of G for a reaction at equilibrium? What... Problem 9RQ: Even if G is negative, the reaction may not occur. Explain the interplay between the thermodynamics... Problem 10RQ: Discuss the relationship between wmax and the magnitude and sign of the free energy change for a... Problem 1ALQ: For the process A(l) A(g), which direction is favored by changes in energy probability? Positional... Problem 2ALQ: For a liquid, which would you expect to be larger, Sfusion or Sevaporation? Why? Problem 3ALQ: Gas A2 reacts with gas B2 to form gas AB at a constant temperature. The bond energy of AB is much... Problem 4ALQ: What types of experiments can be carried out to determine whether a reaction is spontaneous? Does... Problem 5ALQ: A friend tells you, Free energy G and pressure P are related by the equation G = G + RT ln(P). Also,... Problem 6ALQ Problem 7ALQ: Predict the sign of S for each of the following and explain. a. the evaporation of alcohol b. the... Problem 8ALQ: Is Ssurr favorable or unfavorable for exothermic reactions? Endothermic reactions? Explain. Problem 9ALQ: At 1 atm, liquid water is heated above 100C. For this process, which of the following choices (iiv)... Problem 10ALQ: When (if ever) are high temperatures unfavorable to a reaction thermodynamically? Problem 11Q: The synthesis of glucose directly from CO2 and H2O and the synthesis of proteins directly from amino... Problem 12Q: When the environment is contaminated by a toxic or potentially toxic substance (for example, from a... Problem 13Q: Entropy has been described as times arrow. Interpret this view of entropy. Problem 14Q: Human DNA contains almost twice as much information as is needed to code for all the substances... Problem 15Q: A mixture of hydrogen gas and chlorine gas remains unreacted until it is exposed to ultraviolet... Problem 16Q: Consider the following potential energy plots: a. Rank the reactions from fastest to slowest and... Problem 17Q: Ssurr is sometimes called the energy disorder term. Explain. Problem 18Q: Given the following illustration, what can be said about the sign of S for the process of solid NaCl... Problem 19Q: The third law of thermodynamics states that the entropy of a perfect crystal at 0 K is zero. In... Problem 20Q: The deciding factor on why HF is a weak acid and not a strong acid like the other hydrogen halides... Problem 21Q: List three different ways to calculate the standard free energy change, G, for a reaction at 25C.... Problem 22Q: What information can be determined from G for a reaction? Does one get the same information from G,... Problem 23Q: Monochloroethane (C2H5Cl) can be produced by the direct reaction of ethane gas (C2H6) with chlorine... Problem 24Q: At 1500 K, the process I2(g)2I(g)10atm10atm is not spontaneous. However, the process... Problem 25E: Which of the following processes are spontaneous? a. Salt dissolves in H2O. b. A clear solution... Problem 26E: Which of the following processes are spontaneous? a. A house is built. b. A satellite is launched... Problem 27E: Table 16-1 shows the possible arrangements of four molecules in a two-bulbed flask. What are the... Problem 28E: Consider the following illustration of six molecules of gas in a two-bulbed flask. a. What is the... Problem 29E: Consider the following energy levels, each capable of holding two particles: Draw all the possible... Problem 30E: Redo Exercise 29 with two particles A and B, which can be distinguished from each other. Problem 31E: Choose the substance with the larger positional probability in each case. a. 1 mole of H2 (at STP)... Problem 32E: Which of the following involve an increase in the entropy of the system? a. melting of a solid b.... Problem 33E: Predict the sign of Ssurr for the following processes. a. H2O(l) H2O(g) b. I2(g) I2(s) Problem 34E: Calculate Ssurr for the following reactions at 25C and 1 atm. a. C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(l)... Problem 35E: Given the values of H and S, which of the following changes will be spontaneous at constant T and p?... Problem 36E: At what temperatures will the following processes be spontaneous? a. H = 18 kJ and S = 60. J/K b. H... Problem 37E: Ethanethiol (C2H5SH; also called ethyl mercaptan) is commonly added to natural gas to provide the... Problem 38E: For mercury, the enthalpy of vaporization is 58.51 kJ/mol and the entropy of vaporization is 92.92... Problem 39E: For ammonia (NH3), the enthalpy of fusion is 5.65 kJ/mol and the entropy of fusion is 28.9 J/K mol.... Problem 40E: The enthalpy of vaporization of ethanol is 38.7 kJ/mol at its boiling point (78C). Determine Ssys,... Problem 41E: Predict the sign of S for each of the following changes. Assume all equations are balanced. a. b. c.... Problem 42E: Predict the sign of S for each of the following changes. a. K(s) + 12Br2(g) KBr(s) b. N2(g) +... Problem 43E: For each of the following pairs of substances, which substance has the greater value of S? a.... Problem 44E: For each of the following pairs, which substance has the greater value of S? a. N2O (at 0 K) or He... Problem 45E: Predict the sign of S and then calculate S for each of the following reactions. a. 2H2S (g) + SO2(g)... Problem 46E: Predict the sign of S and then calculate S for each of the following reactions. a. H2(g) + 12O2(g) ... Problem 47E: For the reaction C2H2(g)+4F2(g)2CF4(g)+H2(g) S is equal to 358 J/K. Use this value and data from... Problem 48E: For the reaction CS2(g)+3O2(g)CO2(g)+2SO2(g) S is equal to 143 J/K. Use this value and data from... Problem 49E: It is quite common for a solid to change from one structure to another at a temperature below its... Problem 50E: Two crystalline forms of white phosphorus are known. Both forms contain P4 molecules, but the... Problem 51E: Consider the reaction 2O(g)O2(g) a. Predict the signs of H and S. b. Would the reaction be more... Problem 52E: Hydrogen cyanide is produced industrially by the following exothermic reaction:... Problem 53E: From data in Appendix 4, calculate H, S, and G for each of the following reactions at 25C. a. CH4(g)... Problem 54E: The major industrial use of hydrogen is in the production of ammonia by the Haber process:... Problem 55E: For the reaction at 298 K, 2NO2(g)N2O4(g) the values of H and S are 58.03 kJ and 176.6 J/K,... Problem 56E: At 100C and 1.00 atm, H = 40.6 kJ/mol for the vaporization of water. Estimate G for the vaporization... Problem 57E: Given the following data: 2H2(g)+C(s)CH4(g)G=51kJ2H2(g)+O2(g)2H2O(l)G=474kJC(s)+O2(g)CO2G=394kJ... Problem 58E: Given the following data:... Problem 59E: For the reaction SF4(g)+F2(g)SF6(g) the value of G is 374 kJ. Use this value and data from Appendix... Problem 60E: The value of G for the reaction 2C4H10(g)+13O2(g)8CO2(g)+10H2O(l) is 5490. kJ. Use this value and... Problem 61E: Consider the reaction Fe2O3(s)+3H2(g)2Fe(s)+3H2O(g) a. Use Gf values in Appendix 4 to calculate G... Problem 62E: Consider the reaction 2POCl3(g)2PCl3(g)+O2(g) a. Calculate G for this reaction. The Gfvalues for... Problem 63E: Using data from Appendix 4, calculate H, S and G for the following reactions that produce acetic... Problem 64E: Consider two reactions for the production of ethanol:... Problem 65E: Using data from Appendix 4, calculate G for the reaction NO(g)+O3(g)NO2(g)+O2(g) for these... Problem 66E: Using data from Appendix 4, calculate G for the reaction 2H2S(g)+SO2(g)3Srhombic(s)+2H2O(g) for the... Problem 67E: Consider the reaction 2NO2(g)N2O4(g) For each of the following mixtures of reactants and products at... Problem 68E: Consider the following reaction: N2(g)+3H2(g)2NH3(g) Calculate G for this reaction under the... Problem 69E: One of the reactions that destroys ozone in the upper atmosphere is NO(g)+O3(g)NO2(g)+O2(g) Using... Problem 70E: Hydrogen sulfide can be removed from natural gas by the reaction 2H2S(g)+SO2(g)3S(s)+2H2O(g)... Problem 71E: Consider the following reaction at 25.0C: 2NO2(g)N2O4(g) The values of H and S are 58.03 kJ/mol and... Problem 72E: The standard free energies of formation and the standard enthalpies of formation at 298 K for... Problem 73E: Calculate G forH2O(g)+12O2(g)H2O2(g) at 600. K, using the following data:... Problem 74E: The Ostwald process for the commercial production of nitric acid involves three steps:... Problem 75E: Cells use the hydrolysis of adenosine triphosphate, abbreviated as ATP, as a source of energy.... Problem 76E: One reaction that occurs in human metabolism is For this reaction G= 14 kJ at 25c. a. Calculate K... Problem 77E: Consider the following reaction at 800. K: N2(g)+3F2(g)2NF3(g) An equilibrium mixture contains the... Problem 78E: Consider the following reaction at 298 K: 2SO2(g)+O2(g)2SO3(g) An equilibrium mixture contains O2(g)... Problem 79E: Consider the relationship In(K)=HRT+SR The equilibrium constant for some hypothetical process was... Problem 80E: The equilibrium constant K for the reaction 2CI(g)CI2(g) was measured as a function of temperature... Problem 81AE: Using Appendix 4 and the following data, determine S for Fe(CO)5(g).... Problem 82AE: Some water is placed in a coffee-cup calorimeter. When 1.0 g of an ionic solid is added, the... Problem 84AE: Calculate the entropy change for the vaporization of liquid methane and liquid hexane using the... Problem 85AE: As O2(l) is cooled at 1 atm, it freezes at 54.5 K to form solid I. At a lower temperature, solid I... Problem 86AE: Consider the following reaction: H2O(g)+CI2O(g)2HOCI(g)K298=0.090 For Cl2O(g),... Problem 87AE: Using the following data, calculate the value of Ksp for Ba(NO3)2, one of the least soluble of the... Problem 88AE: Many biochemical reactions that occur in cells require relatively high concentrations of potassium... Problem 89AE: Carbon monoxide is toxic because it bonds much more strongly to the iron in hemoglobin (Hgb) than... Problem 90AE: In the text, the equation G=G+RTIn(Q) was derived for gaseous reactions where the quantities in Q... Problem 91AE Problem 92AE: Use the equation in Exercise 79 to determine H and S for the autoionization of water:... Problem 93AE Problem 94AE: Consider the following diagram of free energy (G) versus fraction of A reacted in terms of moles for... Problem 95CWP Problem 96CWP: For rubidium Hvapo=69.0KJ/mol at 686C, its boiling point. Calculate S, q, w, and E for the... Problem 97CWP: Given the thermodynamic data below, calculate S and Ssurr for the following reaction at 25C and 1... Problem 98CWP: Consider the reaction: H2S(g)+SO2(g)3S(g)+2H2O(l) for which H is 233 kJ and S is 424 J/K. a.... Problem 99CWP: The following reaction occurs in pure water: H2O(l)+H2O(l)H3O+(aq)+OH-(aq) which is often... Problem 100CWP Problem 101CWP: Consider the reaction: PCl3(g)+Cl2(g)PCl5(g) At 25C, H = 92.50 kJ. Which of the following statements... Problem 102CWP: The equilibrium constant for a certain reaction increases by a factor of 6.67 when the temperature... Problem 103CP: Consider two perfectly insulated vessels. Vessel 1 initially contains an ice cube at 0C and water at... Problem 104CP: Liquid water at 25C is introduced into an evacuated, insulated vessel. Identify the signs of the... Problem 105CP: Using data from Appendix 4, calculate H, G, and K (at 298 K) for the production of ozone from... Problem 106CP: Entropy can be calculated by a relationship proposed by Ludwig Boltzmann: S=kIn(W) where k = 1.38 ... Problem 107CP: a. Using the free energy profile for a simple one-step reaction, show that at equilibrium K = kf/kr,... Problem 108CP: Consider the reaction H2(g)+Br2(g)2HBr(g) where H = 103.8 kJ/mol. In a particular experiment, equal... Problem 109CP: Consider the system A(g)B(g) at25C. a. Assuming that GAo=8996J/molandGBo=11,718J/mol, calculate the... Problem 110CP: The equilibrium constant for a certain reaction decreases from 8.84 to 3.25 102 when the... Problem 111CP: If wet silver carbonate is dried in a stream of hot air. the air must have a certain concentration... Problem 112CP: Carbon tetrachloride (CCl4) and benzene (C6H6) form ideal solutions. Consider an equimolar solution... Problem 113CP: Sodium chloride is added to water (at 25C) until it is saturated. Calculate the Cl concentration in... Problem 114CP: You have a 1.00-L sample of hot water (90.0C) sitting open in a 25.0C room. Eventually the water... Problem 115CP: Consider a weak acid, HX. If a 0.10-M solution of HX has a pH of 5.83 at 25C. what is G for the... Problem 116IP: Some nonelectrolyte solute (molar mass = 142 g/mol) was dissolved in 150. mL of a solvent (density =... Problem 117IP: For the equilibrium A(g)+2B(g)C(g) the initial concentrations are [A] = [B] = [C] = 0.100 atm. Once... Problem 118IP: What is the pH of a 0. 125-M solution of the weak base B if H= 28.0 kJ and S= 175 J/K for the... Problem 119MP: Impure nickel, refined by smelting sulfide ores in a blast furnace, can be converted into metal from... Problem 7RQ: If you calculate a value for G for a reaction using the values of Gf in Appendix 4 and get a...
Related questions
Concept explainers
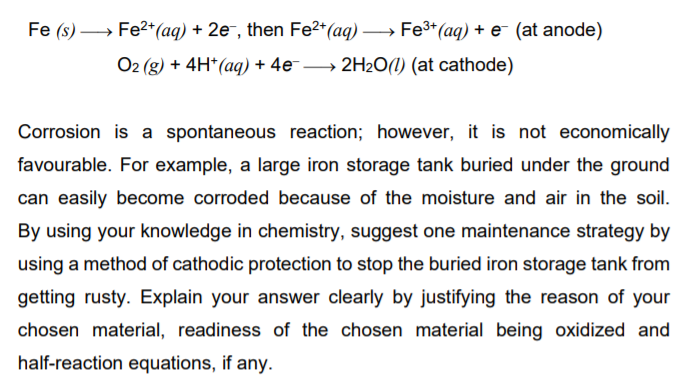
A corrosion process is an example of electrochemistry . A piece of iron (Fe) when exposed to air (oxygen) and humidity undergoes a process of corrosion (the formation of iron(III) oxide) as shown in the two half-reaction equations:
Transcribed Image Text: Fe (s) → Fe2+*(aq) + 2e¯, then Fe2*(aq) → Fe3*(aq) + e¯ (at anode)
O2 (g) + 4H*(aq) + 4e → 2H2O(1) (at cathode)
Corrosion is a spontaneous reaction; however, it is not economically
favourable. For example, a large iron storage tank buried under the ground
can easily become corroded because of the moisture and air in the soil.
By using your knowledge in chemistry, suggest one maintenance strategy by
using a method of cathodic protection to stop the buried iron storage tank from
getting rusty. Explain your answer clearly by justifying the reason of your
chosen material, readiness of the chosen material being oxidized and
half-reaction equations, if any.
Definition Definition Study of chemical reactions that result in the production of electrical energy. Electrochemistry focuses particularly on how chemical energy is converted into electrical energy and vice-versa. This energy is used in various kinds of cells, batteries, and appliances. Most electrochemical reactions involve oxidation and reduction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images