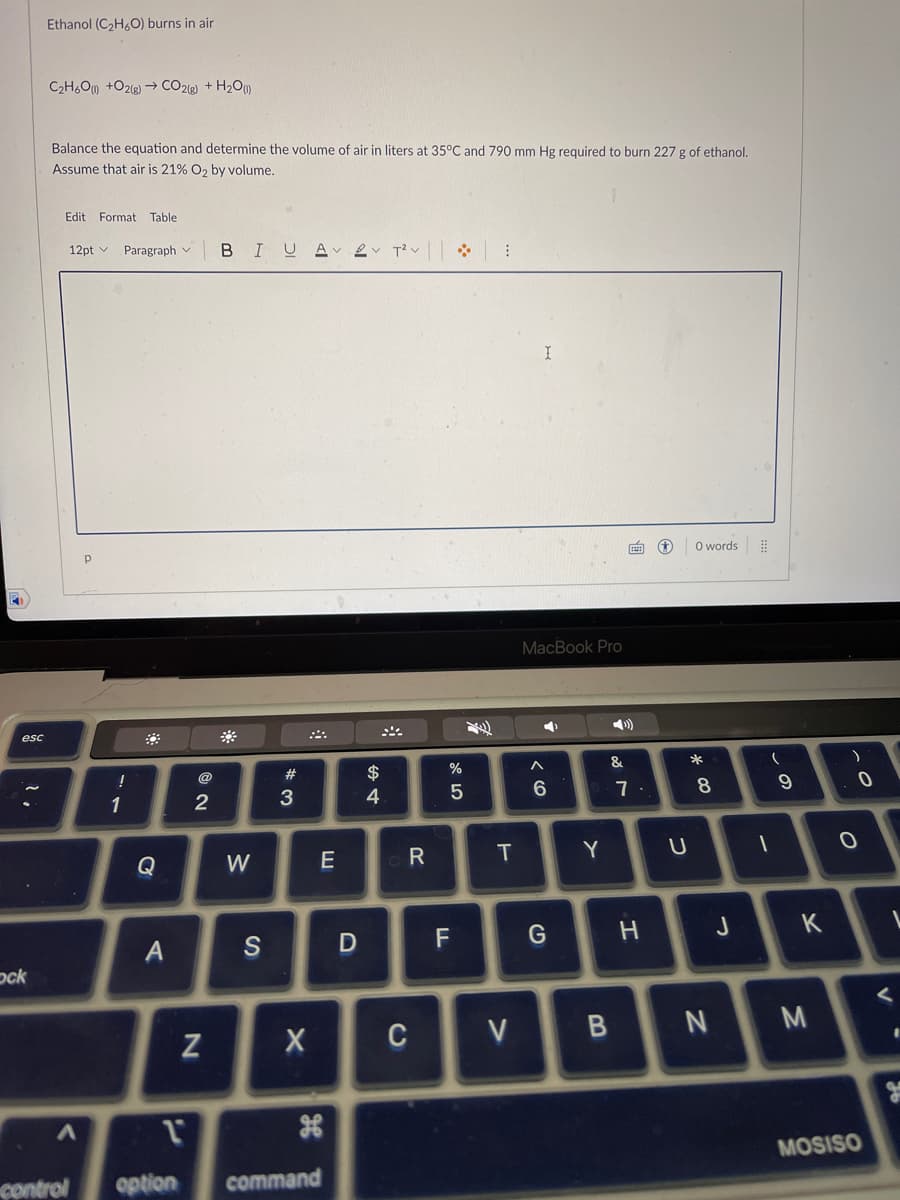
Ethanol (C₂H6O) burns in air C₂H6O(1) +O2(g) → CO2(g) + H₂O (1) Balance the equation and determine the volume of air in liters at 35°C and 790 mm Hg required to burn 227 g of ethanol. Assume that air is 21% O₂ by volume.
Ethanol (C₂H6O) burns in air C₂H6O(1) +O2(g) → CO2(g) + H₂O (1) Balance the equation and determine the volume of air in liters at 35°C and 790 mm Hg required to burn 227 g of ethanol. Assume that air is 21% O₂ by volume.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.11E: What is the value of FP for a sample of gas whose temperature is -33.0 C and volume is 0.0250 L?...
Related questions
Question
Transcribed Image Text:esc
pock
Ethanol (C₂H6O) burns in air
C₂H60) +O2(g) → CO2(g) + H₂O (1)
Balance the equation and determine the volume of air in liters at 35°C and 790 mm Hg required to burn 227 g of ethanol.
Assume that air is 21% O₂ by volume.
Edit Format Table
12pt Paragraph
control
!
1
Q
A
@
2
N
BIU AT²V
W
S
#
3
X
1
option command
E
D
$
4
t
R
C
܀
%
5
F
:
T
V
MacBook Pro
➡
A
6
G
Y
&
B
7.
H
(+)
U
0 words
*00
8
N
J
1
(
9
K
M
)
0
O
MOSISO
go
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning