ethylene glycol are shown here. Surface Tension Viscosity (mPa s) Compound Molecule (mN/m) diethyl ether C2H5OC2H5 17 0.22 acetone 23 0.31 C2H5OC2H5 ethanol 22 1.07 C2H;OH ethylene glycol CH2(OH)CH2(OH) 48 16.1 a. Explain their differences in viscosity in terms of the size and shape of their molecules and their IMFS.
ethylene glycol are shown here. Surface Tension Viscosity (mPa s) Compound Molecule (mN/m) diethyl ether C2H5OC2H5 17 0.22 acetone 23 0.31 C2H5OC2H5 ethanol 22 1.07 C2H;OH ethylene glycol CH2(OH)CH2(OH) 48 16.1 a. Explain their differences in viscosity in terms of the size and shape of their molecules and their IMFS.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 24E: The surface tension and viscosity values for diethyl ether, acetone, ethanol, and ethylene glycol...
Related questions
Question
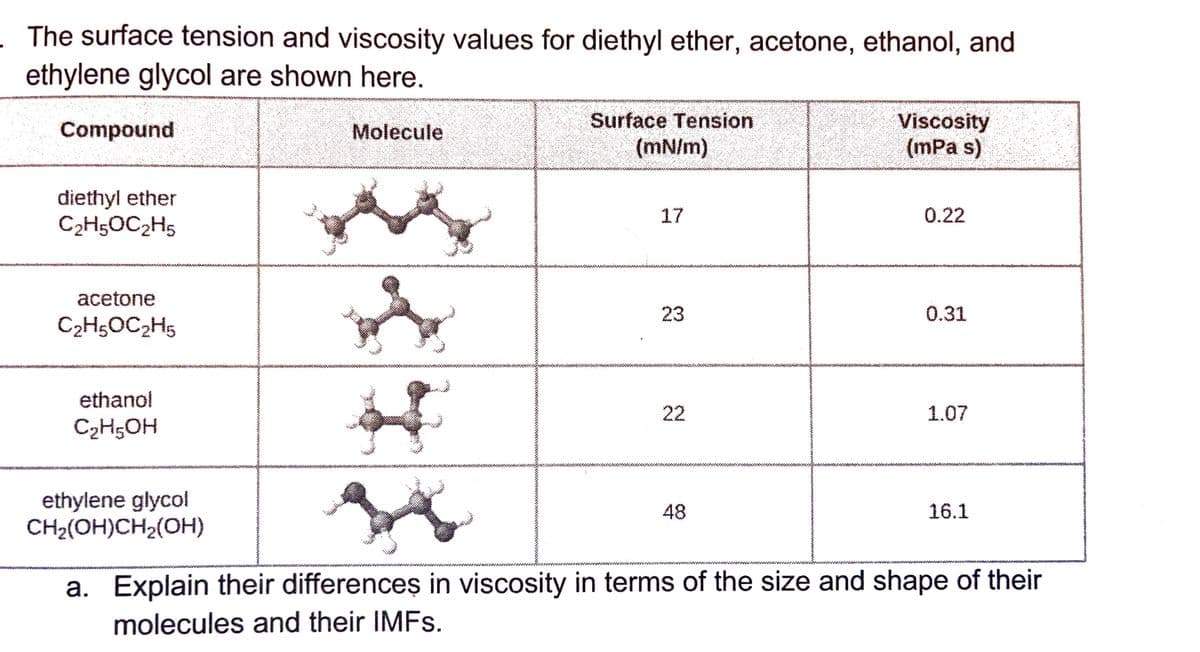
Transcribed Image Text:The surface tension and viscosity values for diethyl ether, acetone, ethanol, and
ethylene glycol are shown here.
Surface Tension
(mN/m)
Viscosity
(mPa s)
Compound
Molecule
diethyl ether
C2H5OC2H5
17
0.22
wwwm
wwwa
wwww
www.w
acetone
23
0.31
C2H5OC2H5
ethanol
22
1.07
C2H;OH
ethylene glycol
CH2(OH)CH2(OH)
48
16.1
a. Explain their differenceș in viscosity in terms of the size and shape of their
molecules and their IMFS.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning