Exam .1. Compute the quantities of lime and soda required to soften raw water containing alkalinity of 220 mg|l; hardness as CaCl, = 40 mg il, and as MgSO, = 60 mg/1. Water required to be treated is 1 million litres. %3D
Q: What volume of 0.205 mol L-1 NaOH solution is needed to just completely neutralize a 0.432 mol L-1 s...
A:
Q: 1. A solution is prepared by dissolving 12 grams of turmeric powder in 350 grams of water. Calculate...
A:
Q: Time (s) [NO2] (mol/L) 0 0.440 1.55×103 0.418 3.88×103 0.389 5.81×103 0.368 1.16×104 0.317 2.32×104...
A:
Q: Fisher Ball-and-stick Haworth Chair/ Envelope Conformation [2] Complete name Projection structure re...
A: Ans. [1]α-D-mannopyranose: Ball-and-stick structure [2] Chair/envelope conformation: [3] D-psicose...
Q: Hydrogenation of one mole of the triene A with one mole of H, gives isomeric dienes having molecular...
A: Woodward-Fieser Rules is used for Calculating the λmax of Conjugated Dienes and Polyenes.
Q: 11. From the balanced molecular equations, write the complete ionic and net ionic equations for the ...
A: To write a complete ionic and net ionic equations the following steps should be followed - 1. ...
Q: Calculate the report the volumes of weak acid, conjugate base and distilled water need to prepare yo...
A: Solution: The buffer: Acetic acid (CH3COOH) and Sodium acetate (NaCH3COO).The (CH3COOH) is weak acid...
Q: identify which of the two has the fastest and slowest reaction, and what could be the reasons betwee...
A:
Q: Put the species in order, from strongest reducing agent (#1) to the weakest reducing agent (#6). You...
A:
Q: State whether true or false and briefly explain a. Student A would like to compute for the total he...
A: The given statement is false. It needs Hfusion plus some extra heat.
Q: 29. Great Lakes Chemical Company produces bromine, Br2, from bromide salts such as NaBr, in Arkansas...
A: Answer: This is question is based on the reactivity order of non-metals.
Q: Draw the molecular mass fragments for the following m/z rations. Show all the atoms (including) all ...
A:
Q: An ideal gas in a cylinder fitted with a piston expands at constant temperature from a pressure of 5...
A:
Q: Q 15: Identify the Intermolecular forces (IMF) present in the following molecules: a. PH3 b. CH3CH2O...
A:
Q: State whether true or false and briefly explain a. The reaction: X(l) → Y(g) + Z(g) results to posi...
A: A positive work is said to be done if the work is done on the system to change it or modify it. A ne...
Q: A solution has a [OH−] = 3.4 × 10−5 M at 25 °C. What is the [H3O+] of the solution?
A:
Q: 1. An aqueous solution is prepared that is initially 0.100 M Cdl2. After the equilibrium is establis...
A: “Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for y...
Q: U STATES OF MATTER Understanding how average molecular speed scales wit Rank the samples of gas desc...
A:
Q: Which of the following reactions require different reagents for terminal vs internal alkynes O reduc...
A:
Q: thermometer A 55.4 g sample of aluminum, which has a specific heat capacity of 0.897 J'g , is put in...
A: Given that, For Al, m=55.4 g, Cp=0.897 J g-1°C-1 , T1= ? , T2=20.7 °C For water, m= 300.0 g, Cp= 4...
Q: A reaction has a rate constant of 0.00205 s–1. What is the half-life (in min to two decimal places) ...
A:
Q: Which of the following chemicals would have a higher boiling point?
A: Boiling point is the temperature at which boiling point of solution becomes equal to atmospheric pre...
Q: NH(CH32 A
A:
Q: What mass of excess reactant remains at the end of the reaction if 90.0 g of SO2 are mixed with 100....
A:
Q: Draw the titration curve that would result if 25 mL of a 0.2 M HA (monoprotic acid, ka = 1.80 x 10-5...
A: Answer: In this question we have to find out the pH of two types of solutions: 1. pH of the solution...
Q: A sample of polystyrene, which has a specific heat capacity of 1.880 J-g .°c', is put into a calorim...
A: Given data,Specific heat capacity of polystyrene=1.880J.g-1.oC-1Mass of water=250.0gInitial temperat...
Q: reaction compound concentration expected change in concentration vessel CH,CH, NH, 0.79 M f increase...
A: A question based on equilibrium concept that is to be accomplished.
Q: What is electronegativity?
A: The tendency to attract shared pair of electron is called electronegativity. Fluorine is most elect...
Q: Draw the structure of (Z)-2,3-dichloro-4-methyl-2-hexene.
A: For E nomenclature, more prior group are on the opposite side . For Z nomenclature ,more prior gro...
Q: Calculate the electronegativity differences and determine the bond type for each of the following bo...
A: Since you have asked a question with multiple sub-parts, as per our company guidelines we are suppos...
Q: Which gas has the greatest density? Question 4 options: A) 2.3 mol of He at 1.2 atm an...
A:
Q: Omol
A:
Q: What is the pH of a 0.150 M NH4Cl solution? Kb of NH3 = 1.76 × 10−5
A: Given, Concentration of NH4Cl solution (C) = 0.150 M Kb for NH3 = 1.76 × 10-5 pH of a NH4Cl solution...
Q: Which of the following would be heterogeneous? A mixture of sand and water. A mixture ...
A: A heterogeneous mixture is a mixture with non-uniform composition or we can say it contains two diff...
Q: potassium phosphite
A: This is a multiparts question. According to guidelines in multiparts question we can solve only two ...
Q: 7. Although Huckel's rule strictly applies only to monocyclic compounds, it does appear to have appl...
A: For a compound being aromatic,there is some rule which is known as Huckel's rule of aromaticity.
Q: 1. Use the internet and your data table to figure out which of the following COVALENT NETWORK SOLIDS...
A: Answer :
Q: If the proportion of particles with energy is greater than activation energy, the reaction will O a....
A: Given statement is : If the proportion of particles with energy is greater than activation energy, ...
Q: the amount o
A: Since you have asked multiparts, we will solve the first three subparts for you. If you want any spe...
Q: The N and P recommendation for a corn crop is 170 Ibs N/ac and 85 lbs P205/ac. How much anhydrous am...
A: Reccomemded nitrogen = 170 lbs = 77110.7 g No of moles = Mass/Molar mass Moles of N recommended = 77...
Q: Exactly 75.00 mL of a 0.3132 M solution of Na2SO3 were treated with 150.0 mL of 0.4025 M HClO4 and b...
A: Given, Exactly 75.00 mL of a 0.3132 M solution of Na2SO3 were treated with 150.0 mL of 0.4025 M HCl...
Q: Nitrogen and hydrogen react to form ammonia, like this: N2(9)+3H,(g) → 2 NH;(g) The reaction is exot...
A: Le Chatelier's principle: The change in concentration, pressure, volume and temperature of a system ...
Q: The structure is an ethanolamine plasmalogen. Test for unsaturation were done. What are the results ...
A: When a molecule has carbon and hydrogen without any other specific atoms is termed as a hydrocarbon....
Q: Acetanilide 14,3°C compound identity literature m.p. of compound experimental m.p. of your impure sa...
A: Solution- 1a) Let us consider the given data. The compound identity = Acetanilide The literature mel...
Q: The rate of a chemical reaction a. is always increased with increasing temperatures. b. is always de...
A: Chemical kinetics can be defined as the branch of chemistry that deals with rates of chemical reacti...
Q: 5) What is the mass of Ca(OH)2 should be present in 0.250 L of solution to obtain a solution to obta...
A:
Q: A sample of mixture of CaCO3(s) decomposed: CACO3(9) CaO(s) Сао, + 2NaHCO3(s) Na,C
A:
Q: Hello, sorry I think I was a bit bland with the question. In the periodic descriptions attached belo...
A: A question based on periodic properties that is to be accomplished.
Q: Calculate the standard free energy change (kJ/mol) for the reaction shown at a temperature of 298.15...
A: Consider the given information is as follows; ∆Hrxn=-2984.0 kJ/mol ∆Srxn=-961.5 J/mol-K = -0.9615 kJ...
Q: Suppose a 500. mL flask is filled with 0.40 mol of N, and 0.90 mol of NH3. This reaction becomes pos...
A: volume of flask = 500 mL Initial moles of N2 and NH3 are 0.40 and 0.90 moles respectively.
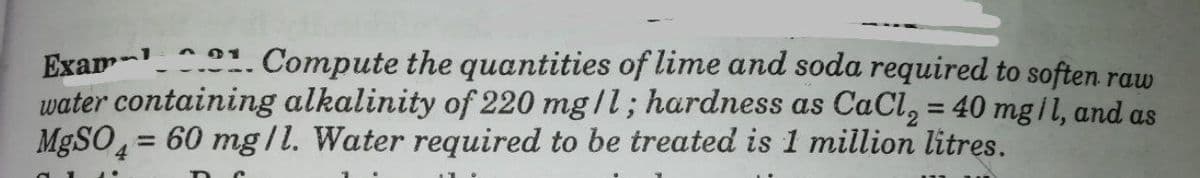
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

- 1. 1093-g sample of impure Na2CO3 was analyzed by residual precipitimetry. After adding 50.00 mL of 0.06911 M AgNO3, the sample was back-titrated with 0.05781 M KSCN, requiring 27.36 mL to reach the endpoint. The percentage Na2CO3 (MW = 106.0 g/mole) in the tested sample is ________ % ? Note: Express final answer using least number of significant figures. 2. The alkalinity of natural waters is usually controlled by OH- (MW = 17.01 g/mole), CO3-2 (MW = 60.01 g/mole), and HCO3- (MW = 61.01 g/mole), which may be present singularly or in combination. Titrating a 10.0-mL sample to a phenolphthalein endpoint requires 38.12 mL of a 0.5812 M solution of HCl, and an additional 18.67 mL of the same titrant to reach the methyl orange endpoint. The composition of the sample is _________% CO3-2 and ___________ % OH- Note: Express final answers using least number of significant figures.A 15.00 g sample containing mixed alkali and other inert components was dissolved and diluted to 300 mL with water. A 20 mL aliquot was titrated with 5.02 mL of 0.5352 M HCl to reach PHP endpoint. Another 20 mL aliquot was titrated to the BCG endpoint, using up 18.87 mL of titrant in the process. Note: Answer in two decimal places only. If there is no answer, type in 0.00. Use the molar masses 105.989 ?/??l ??2cO3 and 84.007 g/mol NaHCO3 indicated. The volume of titrant needed to neutralize NaOH is _________ mL.The volume of titrant needed to neutralize Na2CO3 is _____________ mL.The volume of titrant needed to neutralize NaHCO3 is _____________ mL. The mass of NaOH is ___________ g.The mass of Na2CO3 is __________ g.The mass of NaHCO3 is __________ g. The percent weight of NaOH is __________ %.The percent weight of Na2CO3 is __________ %.The percent weight of NaHCO3 is __________ %.A water sample is approximated to have a total hardness equal to 120.0ppm. How many milliliters of 0.01000N EDTA will be needed to titrate 250.00mL of the said water sample?(MM CaCO3=100.09g/mol). ** Need asap pls. Thanks.
- 20.0 mL water sample was titrated with 0.0100 M EDTA solution that required 14.50 mL for CaCO3 hardness. The total hardness of the water sample in terms of CaCO3 (MW = 100.1 g/mole) in parts per million is _________? Note: Express final answer using least number of significant figures. 2. If 35.2 mL of EDTA solution is required to reach the endpoint with 0.3120-g of primary standard grade CaCO3 (MW = 100.1 g/mole), the normal concentration of the EDTA (MW = 292.24) solution is _________ N?find the total hardness for water of lake contains (9.83 x10* ( m) of hco3, (1.25x 10 m) of mg*2,(1.25x10*'m) of ca*2 ,and (0.001 m) co,5. it has a ph value of 10The buret was filled with 0.100 M HCl solution. Then was transferred in a 25.0 mL of saturated calcium hydroxide solution (2g of calcium hydroxide per 100 ml of water) in two separate E-flasks. Then 2 drops of phenolphthalein was added to each flask Titration data for the determination of solubility and Ksp of calcium hydroxide: Trial 2: Final Buret reading (ml)-26.10; Initial Buret reading (ml)- 19.80; Temperature (Celcius)- 25 Voume of HCl used: Trial 2- 6.30mL 1. Compute for the moles of H+ used and the moles of OH- present. moles of H+ used = (concentration of HCl) × (volume of HCl used)moles of OH- = moles of H+ used 2. Construct an ICE table for the reaction.3. Calculate the molar solubility (in mol/L) of OH- and Ca2+.4. Determine the solubility of Ca(OH)2 in g/L. (MM of Ca(OH)2 = 74.096 g/mol). 5. Calculate the Ksp of Ca(OH)26. Compute for the percent error of the experimental value for Ksp of Ca(OH)2 with the literature…
- A sample of water from a river was analyzed by titrating a 125 mL aliquot with 0.0210 M EDTA, consuming 22.52 mL. Express the hardness of the water in ppm of CaCO3.Rinse and fill the buret with 0.100 M HCl solution. Transfer accurately 25.0 mL of saturated calcium hydroxide solution (2g of calcium hydroxide per 100 ml of water) into two separate E-flasks. Add 2 drops of phenolphthalein to each flask and titrate each solution until the pink color disappears. Voume of HCl used: Trial 1= 6.80ml; Trial 2 = 6.30mL Determination of solubility and Ksp of calcium hydroxide 2. Compute for the moles of H+ used and the moles of OH- present. moles of H+ used = (concentration of HCl) × (volume of HCl used)moles of OH- = moles of H+ used 3. Construct an ICE table for the reaction.4. Calculate the molar solubility (in mol/L) of OH- and Ca2+.5. Determine the solubility of Ca(OH)2 in g/L. (MM of Ca(OH)2 = 74.096 g/mol)6. Calculate the Ksp of Ca(OH)2.7. Compute for the percent error of the experimental value for Ksp of Ca(OH)2 with the literature value.(Ksp of Ca(OH)2 = 8.0 × 10-6 at 25°C)The water hardness of tap water has been determined to be 120 (= 120 mg CaCO3 per 1 L of water). After the tap water has run through the ion exchange column, titration of a 20.00 mL sample used only 0.70 mL of 0.0100 M EDTA, to reach the endpoint. Calculate the effectiveness of the ion exchange column: what is the % of the calcium, removed by the ion exchange column? Provide your answer in % and without decimal. ( Please type answer note write by hend)
- The buret was filled with 0.100 M HCl solution. Then was transferred in a 25.0 mL of saturated calcium hydroxide solution (2g of calcium hydroxide per 100 ml of water) in two separate E-flasks. Then 2 drops of phenolphthalein was added to each flask Titration data for the determination of solubility and Ksp of calcium hydroxide: Trial 1: Final Buret reading (ml)-19.80; Initial Buret reading (ml)- 13.00; Temperature (Celcius)- 25 Trial 2: Final Buret reading (ml)-26.10; Initial Buret reading (ml)- 19.80; Temperature (Celcius)- 25 Voume of HCl used: Trial 1- 6.80ml; Trial 2- 6.30mL 1. Compute for the moles of H+ used and the moles of OH- present. moles of H+ used = (concentration of HCl) × (volume of HCl used)moles of OH- = moles of H+ used 2. Construct an ICE table for the reaction.3. Calculate the molar solubility (in mol/L) of OH- and Ca2+.4. Determine the solubility of Ca(OH)2 in g/L. (MM of Ca(OH)2 = 74.096 g/mol). 5.…PLEASE TRY TO ANSWER BOTH IF YOU CAN'T THEN JUST ANSWER 5 PLEASE 5. In our experiment, we took 5.00 ml of unknown solution consisting of Ca2+, Mg2+ to a 250 ml flask, add 30 mL of water , buffer ph 10, a few drops of indicator, and titrated using 20.00 mL of 0.04 M standard EDTA. What is molarity of unknown, M (Ca2+, Mg2+)? What effect did adding KCl to the solutions have on the solubility of KHT? and on the solubility product constant of KHT? Select one. The solubilty of KHT is? increased / decreased / no change and on the solubility product constant of KHT? increased / decreased / no change.Need solution to all parts if not answered I'll downvote solution Find the pH during the titration of 20.00 mL of 0.1910 M benzoic acid, C6H5COOH (Ka = 6.3 10-5), with 0.1910 M NaOH solution after the following additions of titrant. (a) 0 mL (b) 10.00 mL (c) 15.00 mL (d) 20.00 mL (e) 25.00 mL



