Examine the figure below, which compares the energetics of a catalyzed and uncatalyzed reaction during the progress of the reaction from substrate (S) to product (P). The highest peak in such a diagram corresponds to the transition state, which is an unstable, high-energy arrangement of substrate atoms that is intermediate between substrate and product. The free energy required to surmount this barrier to the reaction is termed the activation energy. Enzymes function by lowering the activation energy, thereby allowing a more rapid approach to equilibrium.
Examine the figure below, which compares the energetics of a catalyzed and uncatalyzed reaction during the progress of the reaction from substrate (S) to product (P). The highest peak in such a diagram corresponds to the transition state, which is an unstable, high-energy arrangement of substrate atoms that is intermediate between substrate and product. The free energy required to surmount this barrier to the reaction is termed the activation energy. Enzymes function by lowering the activation energy, thereby allowing a more rapid approach to equilibrium.
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
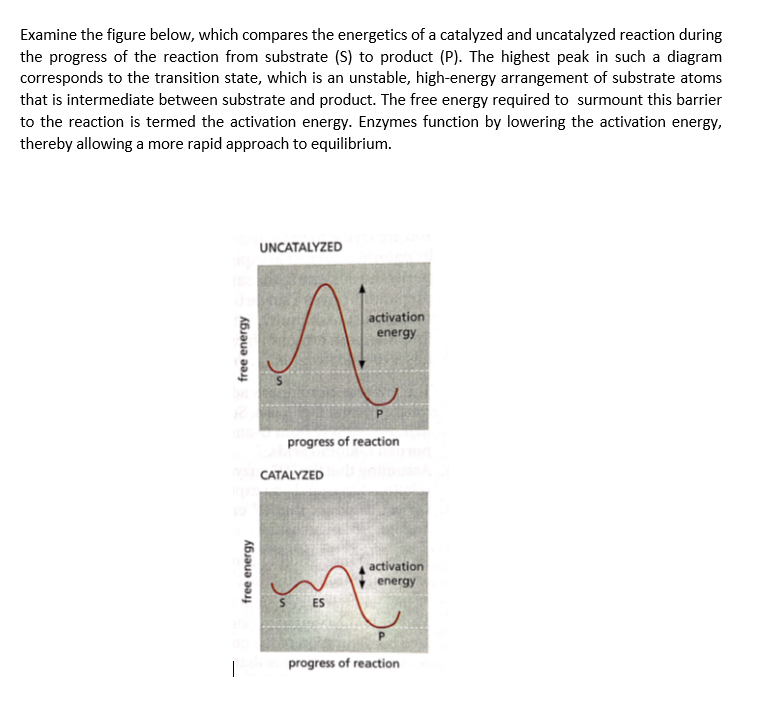
Transcribed Image Text:Examine the figure below, which compares the energetics of a catalyzed and uncatalyzed reaction during
the progress of the reaction from substrate (S) to product (P). The highest peak in such a diagram
corresponds to the transition state, which is an unstable, high-energy arrangement of substrate atoms
that is intermediate between substrate and product. The free energy required to surmount this barrier
to the reaction is termed the activation energy. Enzymes function by lowering the activation energy,
thereby allowing a more rapid approach to equilibrium.
UNCATALYZED
activation
energy
progress of reaction
CATALYZED
activation
energy
S ES
|
progress of reaction
free energy
free energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON