Experiment: Extraction of Eugenol For analysis of medicinal values, the essential constituents are m-Eugenol i.e. 3-Allyl-6- methoxyphenol (69.43%), Eugenol acetate (10.78%), Caryophyllene (6.80%), 2-Pentanone (7.78%) etc. Mass of Cloves: 25.021 grams Mass of Eugenol Recovered: 10.560 grams Mass of Acetyl Eugenol Recovered: 1.416 grams
Experiment: Extraction of Eugenol For analysis of medicinal values, the essential constituents are m-Eugenol i.e. 3-Allyl-6- methoxyphenol (69.43%), Eugenol acetate (10.78%), Caryophyllene (6.80%), 2-Pentanone (7.78%) etc. Mass of Cloves: 25.021 grams Mass of Eugenol Recovered: 10.560 grams Mass of Acetyl Eugenol Recovered: 1.416 grams
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter5: Distillation
Section: Chapter Questions
Problem 10Q
Related questions
Question
please help calculate 1-3 using given data
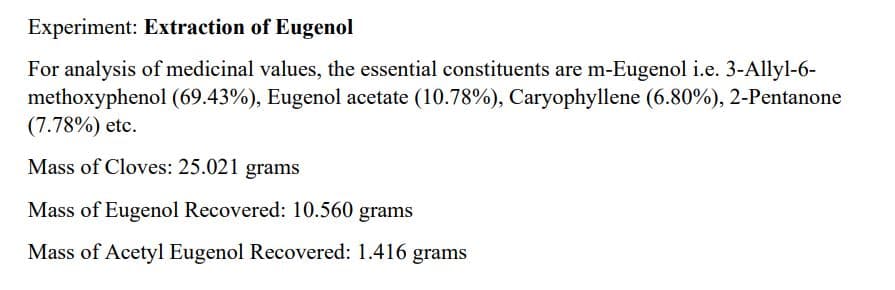
Transcribed Image Text:Experiment: Extraction of Eugenol
For analysis of medicinal values, the essential constituents are m-Eugenol i.e. 3-Allyl-6-
methoxyphenol (69.43%), Eugenol acetate (10.78%), Caryophyllene (6.80%), 2-Pentanone
(7.78%) etc.
Mass of Cloves: 25.021 grams
Mass of Eugenol Recovered: 10.560 grams
Mass of Acetyl Eugenol Recovered: 1.416 grams
![Calculations
1. Calculate the percent recovery of eugenol [(mass eugenol/mass cloves)x100%].
2. Calculate the percent recovery of acetyleugenol.
3. Calculate the percent recovery of both acetyleugenol and eugenol.
Questions
1. Describe in words where the eugenol and the acetyleugenol are during each of the
extraction steps. Include separation and recovery steps. Put the picture above into
your own words.
2. What structural difference allows the separation of eugenol from acetyleugenol?
Draw the reaction of eugenol with NaOH and show the product obtained.
3. Do the online quiz for assigning spectra. Do not scan in spectra!
4. What spectral features (from all spectra) allow you to distinguish between eugenol
and acetyleugenol?](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc02bfde4-a78d-4551-93f6-03d589a5c8dc%2Fdb305d81-bc41-46fb-8e98-8833b7197269%2Ffyait5g_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Calculations
1. Calculate the percent recovery of eugenol [(mass eugenol/mass cloves)x100%].
2. Calculate the percent recovery of acetyleugenol.
3. Calculate the percent recovery of both acetyleugenol and eugenol.
Questions
1. Describe in words where the eugenol and the acetyleugenol are during each of the
extraction steps. Include separation and recovery steps. Put the picture above into
your own words.
2. What structural difference allows the separation of eugenol from acetyleugenol?
Draw the reaction of eugenol with NaOH and show the product obtained.
3. Do the online quiz for assigning spectra. Do not scan in spectra!
4. What spectral features (from all spectra) allow you to distinguish between eugenol
and acetyleugenol?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole