Observations: Table 1: Estimation of DO. SI No Volume of water Initial sample (ml) burette Final burette Volume of NazS203 reading (ml) reading (ml) consumed (V2 ml) 100.0 0.0 7.4 7.4 1 100.0 7.4 14.8 7.4 100.0 14.8 22.1 7.3 3 Calculations: Normality of water sample: N¡V1(water sample) =N2V2(Na2S2O3) Ex 7.4 40 N2 XV2 N13 = 1.85 x 10-3 V1 100 N1 = 1.85 × 10-3 Amount of Dissolved oxygen in water sample: Amount of free chlorine in water sample= N1 x equivalent weight of oxygen = 1.85 x 10-3 x 8 g/L = 1.85 x 10-3 x 8 × 103 ppm = 14.8 PPM Amount of free chlorine in water sample= 14.8 PPM
Observations: Table 1: Estimation of DO. SI No Volume of water Initial sample (ml) burette Final burette Volume of NazS203 reading (ml) reading (ml) consumed (V2 ml) 100.0 0.0 7.4 7.4 1 100.0 7.4 14.8 7.4 100.0 14.8 22.1 7.3 3 Calculations: Normality of water sample: N¡V1(water sample) =N2V2(Na2S2O3) Ex 7.4 40 N2 XV2 N13 = 1.85 x 10-3 V1 100 N1 = 1.85 × 10-3 Amount of Dissolved oxygen in water sample: Amount of free chlorine in water sample= N1 x equivalent weight of oxygen = 1.85 x 10-3 x 8 g/L = 1.85 x 10-3 x 8 × 103 ppm = 14.8 PPM Amount of free chlorine in water sample= 14.8 PPM
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
EXPERIMENT :Estimation of Dissolved Oxygen
I want Result & Analysis for this experiment
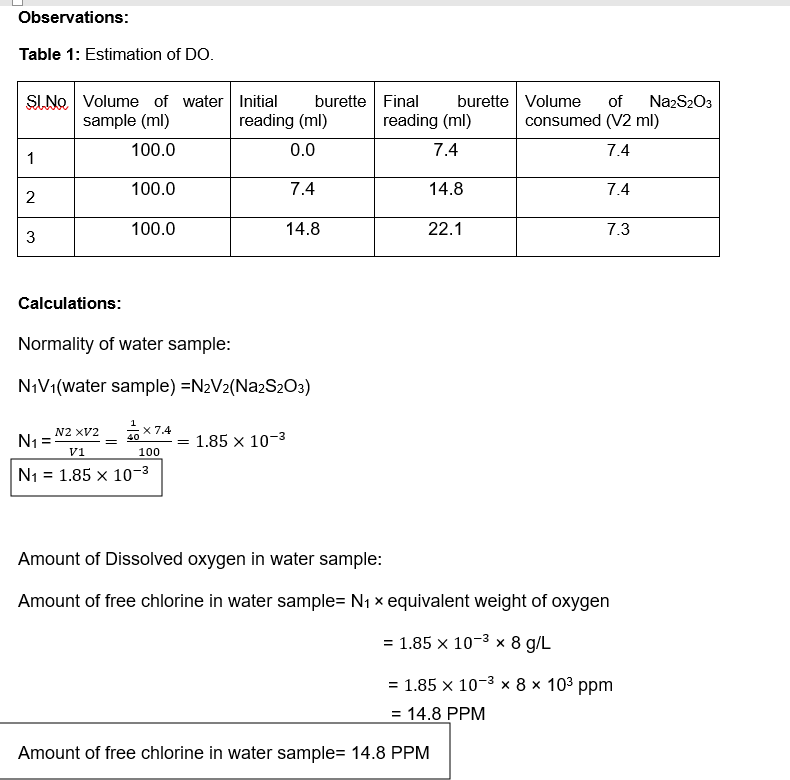
Transcribed Image Text:Observations:
Table 1: Estimation of DO.
SINO Volume of water Initial
sample (ml)
burette Final
burette Volume
of
NazS203
reading (ml)
reading (ml)
consumed (V2 ml)
100.0
0.0
7.4
7.4
1
100.0
7.4
14.8
7.4
2
100.0
14.8
22.1
7.3
Calculations:
Normality of water sample:
N¡V1(water sample) =N2V2(Na2S2O3)
1
N2 XV2
x 7.4
40
N1 =
1.85 x 10-3
V1
100
N1 = 1.85 x 10-3
Amount of Dissolved oxygen in water sample:
Amount of free chlorine in water sample= N1 x equivalent weight of oxygen
1.85 х 10-3 х 8 д/L
%3D
3D 1.85 х 10-3 х 8х 103 рpm
= 14.8 PPM
Amount of free chlorine in water sample= 14.8 PPM
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning