Explain the following figure in detail One by One? Analytes Change during storage Preservation Adsorption to glass wall Precipitation (MO, M(OH)) Add HNO, to pH < 2 Diffusion from plastics Adsorption to plastics Volatilization Metals (M) Use plastic bottle Phthalate ester Oil VOCS Use Teflon or glass bottle Use glass bottle Avoid headspace Add H,SO, to pH < 2 NH, Volatilization Volatilization Add zinc acetate and NaOH to pH > 9 CN Add NaOH to pH > 12 Chemical reaction with Cl; Add ascorbic acid to remove free Cl2 Chemical reaction with Cl; Add Na,S;O, to remove free Cl Volatilization Organic (drinking water) Photochemical degradation Use amber glass container Biodegradation РАН Organic Low pH and temperature; add HgCly to kill bacteria Total phenolics Bacterial degradation Add H,SO, to stop bacterial degradation
Explain the following figure in detail One by One? Analytes Change during storage Preservation Adsorption to glass wall Precipitation (MO, M(OH)) Add HNO, to pH < 2 Diffusion from plastics Adsorption to plastics Volatilization Metals (M) Use plastic bottle Phthalate ester Oil VOCS Use Teflon or glass bottle Use glass bottle Avoid headspace Add H,SO, to pH < 2 NH, Volatilization Volatilization Add zinc acetate and NaOH to pH > 9 CN Add NaOH to pH > 12 Chemical reaction with Cl; Add ascorbic acid to remove free Cl2 Chemical reaction with Cl; Add Na,S;O, to remove free Cl Volatilization Organic (drinking water) Photochemical degradation Use amber glass container Biodegradation РАН Organic Low pH and temperature; add HgCly to kill bacteria Total phenolics Bacterial degradation Add H,SO, to stop bacterial degradation
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.41QAP
Related questions
Question
100%
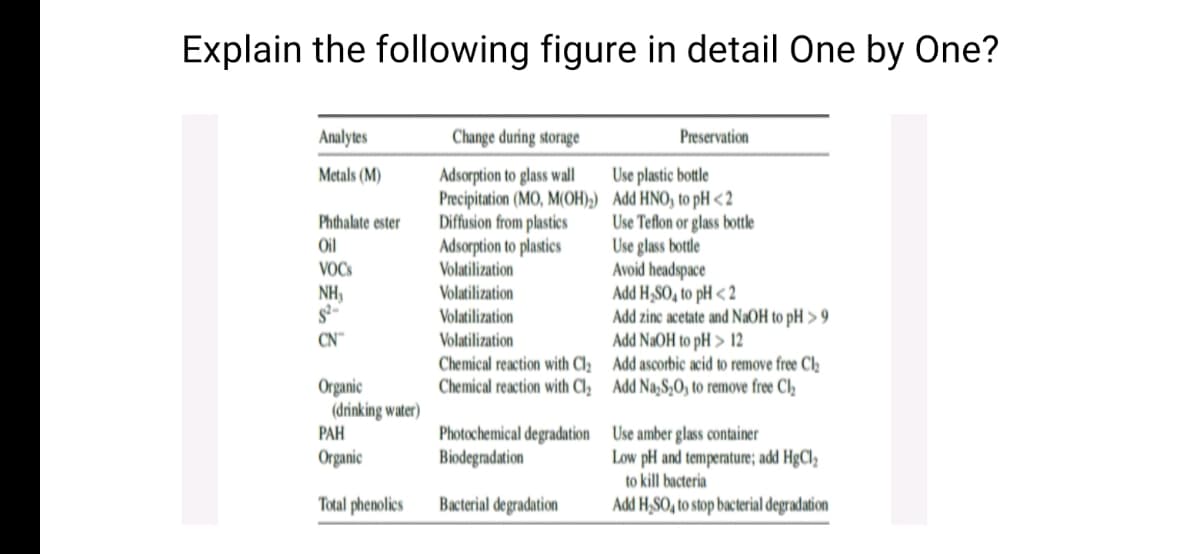
Transcribed Image Text:Explain the following figure in detail One by One?
Analytes
Change during storage
Preservation
Adsorption to glass wall
Precipitation (MO, M(OH);) Add HNO, to pH <2
Diffusion from plastics
Adsorption to plastics
Volatilization
Metals (M)
Use plastic bottle
Phthalate ester
Use Teflon or glass bottle
Use glass bottle
Avoid headspace
Add H,SO, to pH < 2
Oil
VOCS
NH,
s-
CN™
Volatilization
Volatilization
Add zinc acetate and NaOH to pH > 9
Add NaOH to pH > 12
Chemical reaction with Cl2 Add ascorbic acid to remove free Cl2
Chemical reaction with Cl, Add Na,S,O, to remove free Cl,
Volatilization
Organic
(drinking water)
PAH
Photochemical degradation Use amber glass container
Biodegradation
Low pH and temperature; add HgCl2
to kill bacteria
Organic
Total phenolics
Bacterial degradation
Add H,SO, to stop bacterial degradation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

