Fictional, thus not on the periodic table. Lz, a transition metal, forms the compound LzS₂ when bonded to sulfur. What would be the formula of Lz in that same oxidation state bonded to nitrogen? UP 000 H 1 Li Group Na 11 NA K 55 5001 2 Be LZAN L2₂N3 Mg LZN, 12 Rb 38 33 26-1661 26-1564 TO NIH! Cs Cs Ba LZ3N4 LZN LZ3N2 20 15411 4 Den the pruunce of (2-8-) forements 72 and above KEY 139 [DOWN SOWAT e 22 2002 Alom Mess- 12.011 Aco Number 6 Electron Contiguration 2-4 40 RAINING 123 2015 1064 41 POIS With Oxidation Numbers Symbol 6 B 9 10 11 12 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga 21 30 31 2622 124 104 NOME THAC MU MET Ce 58 F4 G C XE Group 26 SEIZE HA 24% 42 43 25 1418148 POTE MIRO 100/8 INIM W Re Os 74 **** 75 FORGETTE Extecker Otton States Fative otomo masos lane based PC 12.000 Ba La Hf Ta 72 ST 516 20141562 25BUS ONION 173 1932-117 76 2019-142 BRI ME M CO PO Fr Ra Ac Rf Db Sg Bh Hs Mt Uun Juu Uub 111 46 112 105 189 109 104 $7 1942-1541 1842 15321592 Note: Mass umbers in protheses masters of the most state of common isotope Pr Nd Pm 59 60 A Th Pa U 90 91 92 27 Edita 145 2-8-18-1 PWA 23 NIES Sm OMICS 62 29 EXIM FORM 48 14-1 78 1 5 13 79 PRIM ***** T 200 13 B Ek Sr Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 201018 81 TH Al FESTE 6 14 164 Np Pu Am Cm Bk Cf 903 97 91 14 32 C Si 49 34- ** DRING 4551 Ir Pt Au Hg Tl Pb Bi 77 P 1912 82 83 1 13631194 *** BER *** 7430 DOP 115 548 15 N P 33 24.358 ERSITET Group S 2016184 P 16 0 34 263 WIL Ge As Se Br Kr M 52 2015156 17 F 17 247 294 34 4532-196 114 The systematic names and symbols for elements of atomic numbers 109 will be used in the approval of trivial names by LIPAC. 25400 S Cl Ar 18 246 315 20107 11:06 18 He FATIM JE 85 15-2016 18 30 Ne T I Xe BEREN 20-10-16 FASHI Bi Po At Rn hom 36 24084 2 FARNING T Eu Gd Tb Dy Ho Er Tm Yb Lu 63 64 65 67 68 70 71 Es Fm Md No Lr 99 100 101 102 103
Fictional, thus not on the periodic table. Lz, a transition metal, forms the compound LzS₂ when bonded to sulfur. What would be the formula of Lz in that same oxidation state bonded to nitrogen? UP 000 H 1 Li Group Na 11 NA K 55 5001 2 Be LZAN L2₂N3 Mg LZN, 12 Rb 38 33 26-1661 26-1564 TO NIH! Cs Cs Ba LZ3N4 LZN LZ3N2 20 15411 4 Den the pruunce of (2-8-) forements 72 and above KEY 139 [DOWN SOWAT e 22 2002 Alom Mess- 12.011 Aco Number 6 Electron Contiguration 2-4 40 RAINING 123 2015 1064 41 POIS With Oxidation Numbers Symbol 6 B 9 10 11 12 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga 21 30 31 2622 124 104 NOME THAC MU MET Ce 58 F4 G C XE Group 26 SEIZE HA 24% 42 43 25 1418148 POTE MIRO 100/8 INIM W Re Os 74 **** 75 FORGETTE Extecker Otton States Fative otomo masos lane based PC 12.000 Ba La Hf Ta 72 ST 516 20141562 25BUS ONION 173 1932-117 76 2019-142 BRI ME M CO PO Fr Ra Ac Rf Db Sg Bh Hs Mt Uun Juu Uub 111 46 112 105 189 109 104 $7 1942-1541 1842 15321592 Note: Mass umbers in protheses masters of the most state of common isotope Pr Nd Pm 59 60 A Th Pa U 90 91 92 27 Edita 145 2-8-18-1 PWA 23 NIES Sm OMICS 62 29 EXIM FORM 48 14-1 78 1 5 13 79 PRIM ***** T 200 13 B Ek Sr Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 201018 81 TH Al FESTE 6 14 164 Np Pu Am Cm Bk Cf 903 97 91 14 32 C Si 49 34- ** DRING 4551 Ir Pt Au Hg Tl Pb Bi 77 P 1912 82 83 1 13631194 *** BER *** 7430 DOP 115 548 15 N P 33 24.358 ERSITET Group S 2016184 P 16 0 34 263 WIL Ge As Se Br Kr M 52 2015156 17 F 17 247 294 34 4532-196 114 The systematic names and symbols for elements of atomic numbers 109 will be used in the approval of trivial names by LIPAC. 25400 S Cl Ar 18 246 315 20107 11:06 18 He FATIM JE 85 15-2016 18 30 Ne T I Xe BEREN 20-10-16 FASHI Bi Po At Rn hom 36 24084 2 FARNING T Eu Gd Tb Dy Ho Er Tm Yb Lu 63 64 65 67 68 70 71 Es Fm Md No Lr 99 100 101 102 103
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 107AP
Related questions
Question
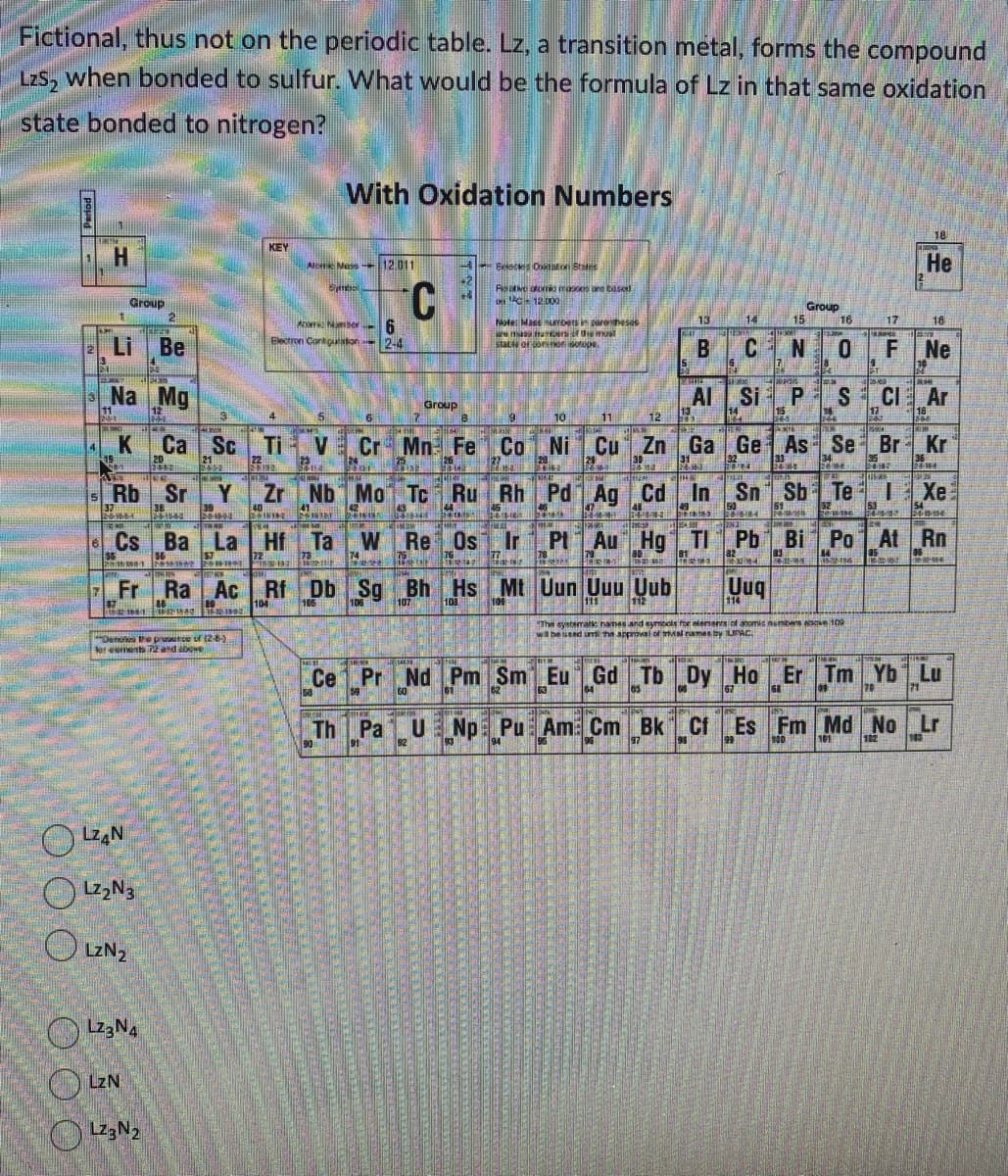
Transcribed Image Text:Fictional, thus not on the periodic table. Lz, a transition metal, forms the compound
LzS₂ when bonded to sulfur. What would be the formula of Lz in that same oxidation
state bonded to nitrogen?
( )
A
000
H
1
Li
11
NA
Na
Group
K
33
26-1661
TONO
17
55
5808001
Rb Sr
38:
26-1564
LZ₂N
LZ₂N3
Cs
Cs Ba
Ba
2
Be
Mg
LZN 2
12
LZ3N4
LZN
20
75411
LZ3N2
56
ST
20161562 250
ONION
PON
Fr Ra Ac
189
4
148
1942-154-12-199 15.12.1992
21
2612
Evk
Den the pruunce of (2-8)
for woments 72 and above
139
DOWNL
TEAT
Y Zr
KEY
22
200
ME
40
RAINING
WA
La Hf Ta
M
72
R 197
Acos Number
Electron Contiguration
104
Alom Mess- 12.011
Rf
Symbol
123
F4
S
2015
431064 SHAR
41
26 WISH
With Oxidation Numbers
173
1932-117
90
Th
6
2-4
C
6
B
9
10
11
12
Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga
29
30
31
24 102
NAME
ECM
FORM
THA
125215
Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te
41
52
42
43
2015 148148
180 M
MIRN
24%
SENS
[1000
49
24-4 14-1 TRIBING 2616184 MUL
74
Pere
Group
1
Extecker Oto Sta
Fative otomo masoes are based
AC 12.000
26
HETER
THE
Note: Mass umbers in partes
ma
state of common isotope
75
HOME
27
Edita
145
3-8-18-1
FRA
76
2017
ME
Db
Db Sg Bh Hs Mt Uun Juu Uub
103
109
111
Ce Pr Nd Pm Sm
568
59
60
23
NIES
Pa U Np Pu
91
92
SC3
OMICS
4
2018
78
1
BUAT
79
HERM
5
ALLE
13
B
13
Al
81
T
FEST
6
14
14
C
Si
Ge
32
PEN
7430
DUBS
115
548
82
[16X144
15
N
Group
P
16
0
34
201
17
F
4501
ESIT
W Re Os Ir Pt Au Hg Tl Pb Bi
Bi Po At Rn
83
77
P
com
192
18
He
25/00
REME
S Cl Ar
14
4538-196
114
"The systematic names and symbols for elements of atomic numbers above 109
will be used in the approval of trivial names by UPAC.
FATER
17
2487
www
As Se Br Kr
TAI
33
24158
315
20107
11:00
18
Ne
30
851
16-201
18
246
53
bonne
2015ING BEREN 24-10-14
36
26-14
2
IXe
FARNING
TEM
Eu Gd Tb Dy Ho Er Tm Yb Lu
63
64
65
67
61
70
71
Am Cm Bk Cf Es Fm Md No Lr
97
91
99
100
101
102
103
FALU
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning