Figure 16.3 shows the solubility of AgNO, in water in units of moles of AgNO, per kilogram of H;O. If 255 g of AgNO, is added to 100 g of water at 95°C and cooled slowly, at what temperature will the solution become saturated? E 16.3 Most solubilities se with increasing temperature, me decrease. Note that the es are not always smooth e different solid hydrates form ifferent temperature ranges. 25 AgNO3 20 AgF 15 NACH;COO NaCl lubility (moles of solute per kg of water)
Figure 16.3 shows the solubility of AgNO, in water in units of moles of AgNO, per kilogram of H;O. If 255 g of AgNO, is added to 100 g of water at 95°C and cooled slowly, at what temperature will the solution become saturated? E 16.3 Most solubilities se with increasing temperature, me decrease. Note that the es are not always smooth e different solid hydrates form ifferent temperature ranges. 25 AgNO3 20 AgF 15 NACH;COO NaCl lubility (moles of solute per kg of water)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 4P: Figure 16.3 shows the solubility of AgNO3 in water inunits of moles of AgNO3 per kilogram of H2O. If...
Related questions
Question
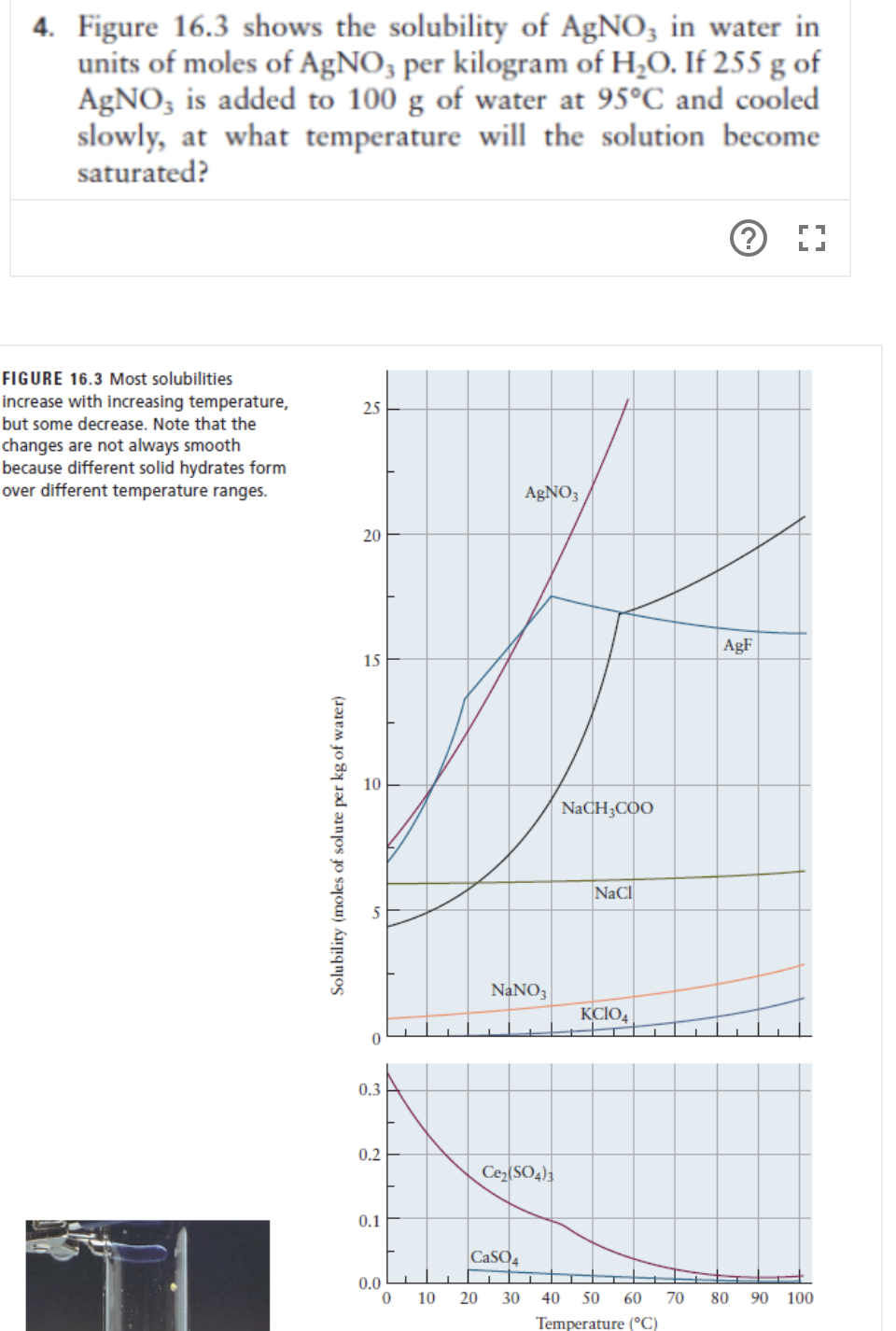
Transcribed Image Text:4. Figure 16.3 shows the solubility of AgNO, in water in
units of moles of AgNO, per kilogram of H,O. If 255 g of
AgNO, is added to 100 g of water at 95°C and cooled
slowly, at what temperature will the solution become
saturated?
FIGURE 16.3 Most solubilities
increase with increasing temperature,
but some decrease. Note that the
25
changes are not always smooth
because different solid hydrates form
over different temperature ranges.
AGNO3
20
AgF
15
NaCH;COO
NaCl
NANO3
KCIO,
0.3
0.2
Ce(SO4);
0.1
CaSO4
0.0
10
20
30
40
50
60
70
80
90 100
Temperature (°C)
Solubility (moles of solute per kg of water)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT