of 1.00 x10-4 M Ag* and 5.00 x10-5 M Cro42, Ag2CrO4 precipita= of 1.0 x10-4 M Ag* and 1.0 x10-4 M I03", Ag(103) precipitate willf y of Ag2CrO4 is lower than the solubility of Ag(1O3). of 0.200 M CrO42- and 0.200 M 103', Ag2(CrO4) will precipitate ove mixture.
of 1.00 x10-4 M Ag* and 5.00 x10-5 M Cro42, Ag2CrO4 precipita= of 1.0 x10-4 M Ag* and 1.0 x10-4 M I03", Ag(103) precipitate willf y of Ag2CrO4 is lower than the solubility of Ag(1O3). of 0.200 M CrO42- and 0.200 M 103', Ag2(CrO4) will precipitate ove mixture.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter13: Electrochemistry
Section: Chapter Questions
Problem 13.90PAE: A chemical engineering student is studying the effect of pH on the corrosion of iron. Ellie...
Related questions
Question
100%
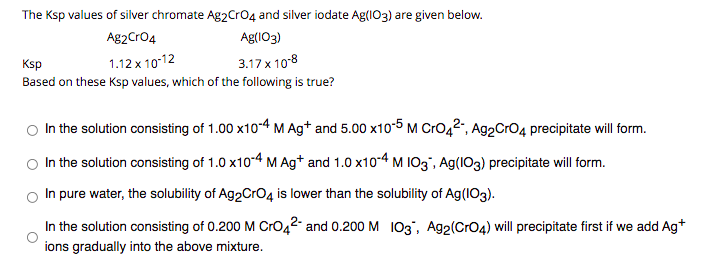
Transcribed Image Text:The Ksp values of silver chromate Ag2Cro4 and silver iodate Ag(IO3) are given below.
Ag2Cro4
Ag(IO3)
1.12 x 1012
3.17 x 10°8
Ksp
Based on these Ksp values, which of the following is true?
In the solution consisting of 1.00 x10-4 M Ag* and 5.00 x10-5 M Cro42", Ag2Cro4 precipitate will form.
In the solution consisting of 1.0 x10-4 MAg* and 1.0 x10-4 M I03", Ag(IO3) precipitate will form.
In pure water, the solubility of Ag2CrO4 is lower than the solubility of Ag(IO3).
In the solution consisting of 0.200 M CrO42- and 0.200 M 103', Ag2(CrO4) will precipitate first if we add Ag*
ions gradually into the above mixture.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning