Figure 23.4 - Mechanism of the Claisen Condensation Reaction. 3 The tetrahedral intermediate expels ethoxide ion to yield a new carbonyl compound, ethyl acetoacetate. 4 But ethoxide ion is a strong enough base to deprotonate ethyl acetoacetate, shift- ing the equilibrium and driving the overall reaction to completion. 5 Protonation of the enolate ion by addition of aqueous acid in a separate step yields the final 3-keto ester product. H3C H3C 17 H3C O=U O=C HH 4 1:CIH O=C Η Η OEt OEt 5 H30+ OEt + EtO™ + EtOH
Figure 23.4 - Mechanism of the Claisen Condensation Reaction. 3 The tetrahedral intermediate expels ethoxide ion to yield a new carbonyl compound, ethyl acetoacetate. 4 But ethoxide ion is a strong enough base to deprotonate ethyl acetoacetate, shift- ing the equilibrium and driving the overall reaction to completion. 5 Protonation of the enolate ion by addition of aqueous acid in a separate step yields the final 3-keto ester product. H3C H3C 17 H3C O=U O=C HH 4 1:CIH O=C Η Η OEt OEt 5 H30+ OEt + EtO™ + EtOH
Chapter23: Carbonyl Condensation Reactions
Section23.7: The Claisen Condensation Reaction
Problem 12P
Related questions
Question
Put more explanation in each step.
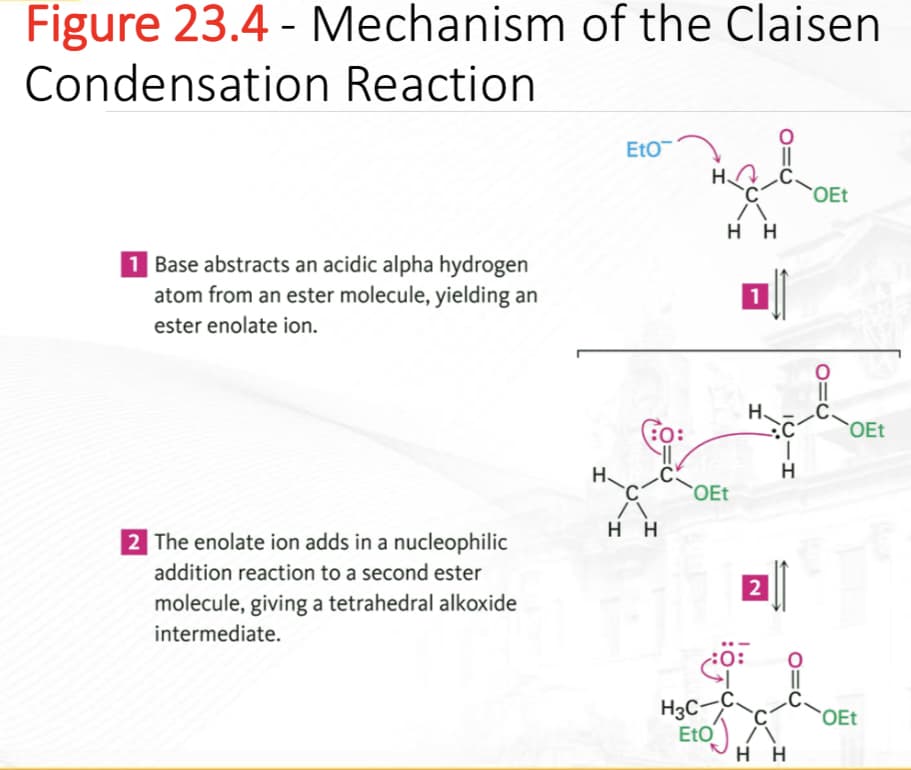
Transcribed Image Text:Figure 23.4 - Mechanism of the Claisen
Condensation Reaction
1 Base abstracts an acidic alpha hydrogen
atom from an ester molecule, yielding an
ester enolate ion.
2 The enolate ion adds in a nucleophilic
addition reaction to a second ester
molecule, giving a tetrahedral alkoxide
intermediate.
H.
EtO
FO:
C
C
A
HH
H.
H3C-
OEt
нн
Eto
30:
1
H.
2
ICIH
HH
OEt
010
C.
OEt
OEt
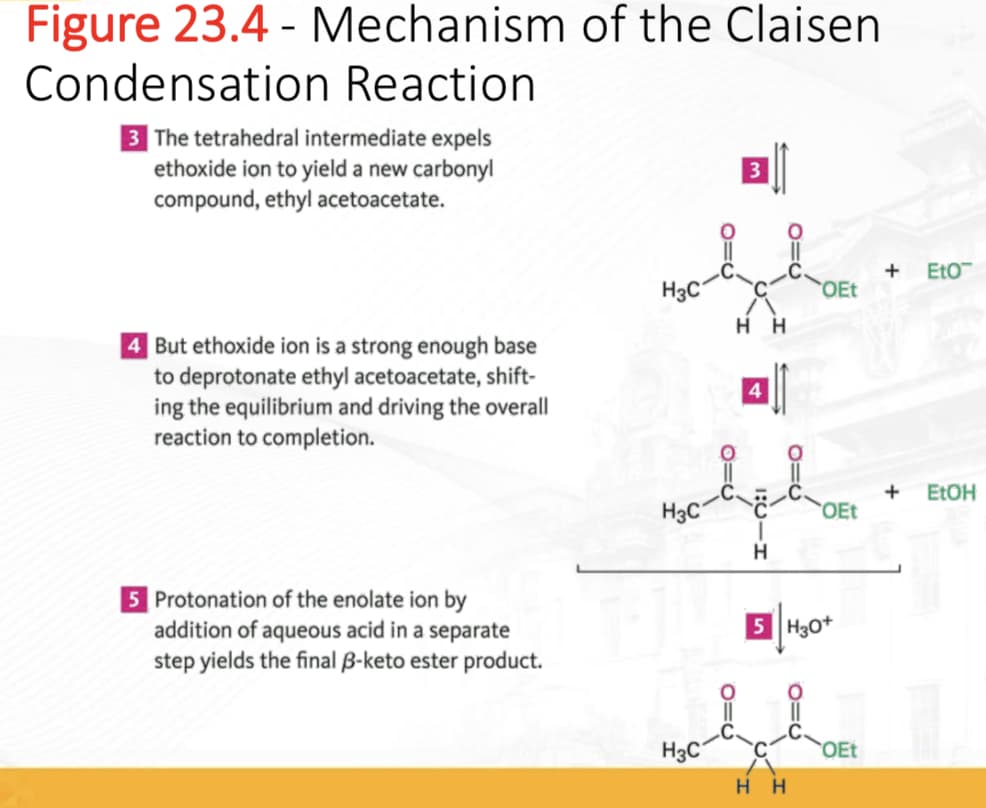
Transcribed Image Text:Figure 23.4 - Mechanism of the Claisen
Condensation Reaction
3 The tetrahedral intermediate expels
ethoxide ion to yield a new carbonyl
compound, ethyl acetoacetate.
4 But ethoxide ion is a strong enough base
to deprotonate ethyl acetoacetate, shift-
ing the equilibrium and driving the overall
reaction to completion.
5 Protonation of the enolate ion by
addition of aqueous acid in a separate
step yields the final 3-keto ester product.
H3C
H3C
H3C
O=U
O=C
HH
4
1:CIH
O=C
Η Η
OEt
OEt
5 H30+
OEt
+ EtO™
+ EtOH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning



Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning