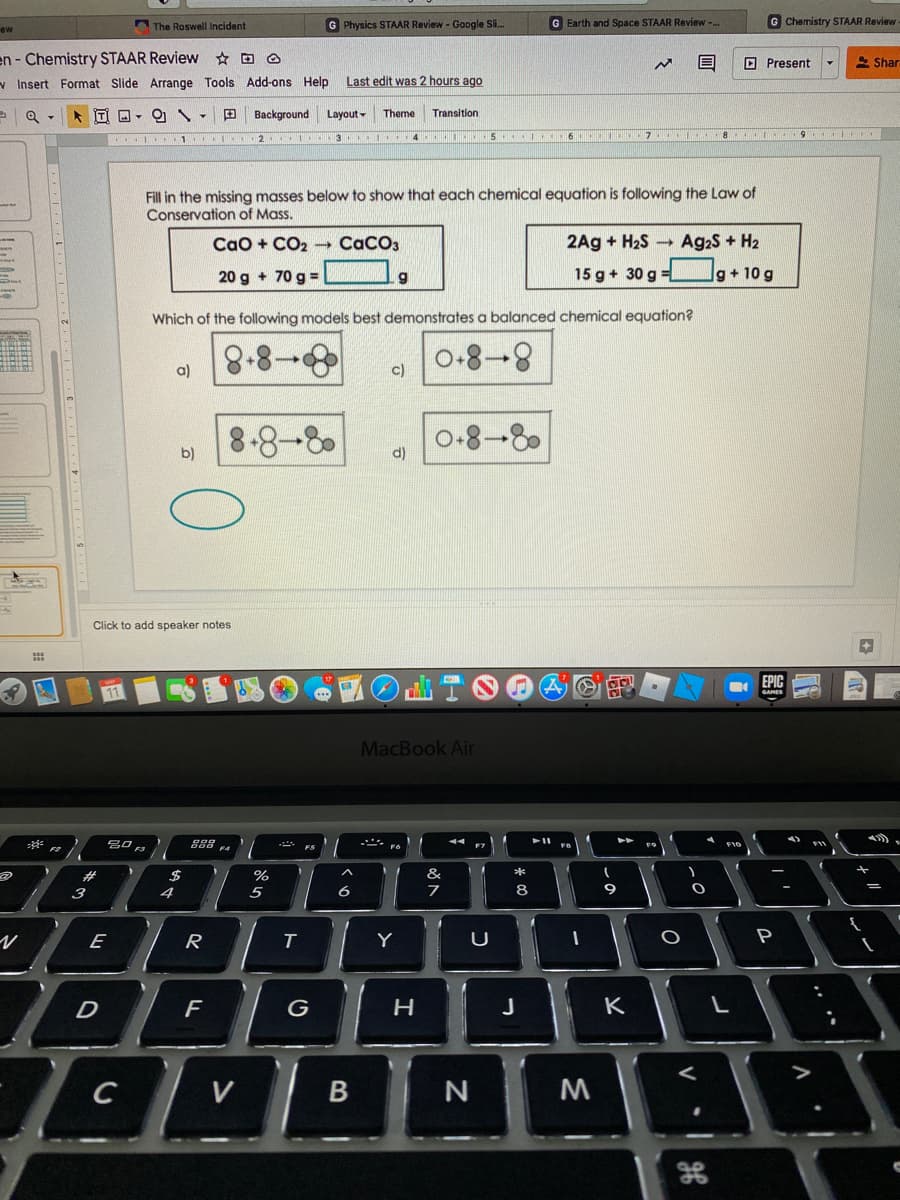
Fill in the missing masses below to show that each chemical equation is following the Law of Conservation of Mass. Cao + CO2 CaCO3 2Ag + H2S Ag.S + H2 20 g + 70 g = 15 g+ 30 g = lg+ 10 g Which of the following models best demonstrates a balanced chemical equation? 8-8- 0-8- a) c) 8-8-8 0-8-8 b) d)
Fill in the missing masses below to show that each chemical equation is following the Law of Conservation of Mass. Cao + CO2 CaCO3 2Ag + H2S Ag.S + H2 20 g + 70 g = 15 g+ 30 g = lg+ 10 g Which of the following models best demonstrates a balanced chemical equation? 8-8- 0-8- a) c) 8-8-8 0-8-8 b) d)
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter9: Pre-quantum Mechanics
Section: Chapter Questions
Problem 9.18E
Related questions
Question
Transcribed Image Text:A The Roswell Incident
G Physics STAAR Review - Google Sli.
G Earth and Space STAAR Review -.
G Chemistry STAAR Review-
ew
en - Chemistry STAAR Review ☆ D O
O Present
* Shar
v Insert Format Slide Arrange Tools Add-ons Help
Last edit was 2 hours ago
T O - O -
田
Background Layout-
Theme
Transition
I 1 IN 2 I 3 I 4 1 S I 6 7 8 9
Fill in the missing masses below to show that each chemical equation is following the Law of
Conservation of Mass.
Cao + CO2 CaCO3
2Ag + H2S
Ag2S + H2
20 g + 70 g =
15 g + 30 g =
lg+ 10 g
Which of the following models best demonstrates a balanced chemical equation?
a)
c)
8-8-8
0-8-8
b)
d)
Click to add speaker notes
+
EPIC
MacBook Air
g88 4
%2#
&
*
3
4
5
6
8
E
Y
P
F
G
H
J
K
C
V
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning
