Combustion of hydrocarbons such as methane (CH) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of gaseous methane into gaseous carbon dioxide and gaseous water. D-0 2. Suppose 0.250 kg of methane are burned in air at a pressure of exactly 1 atm and a temperature of 12.0 °C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits.
Combustion of hydrocarbons such as methane (CH) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of gaseous methane into gaseous carbon dioxide and gaseous water. D-0 2. Suppose 0.250 kg of methane are burned in air at a pressure of exactly 1 atm and a temperature of 12.0 °C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 44AP: A possible practical way to eliminate oxides of nitrogen(such as NO2 ) from automobile exhaust gases...
Related questions
Question
100%
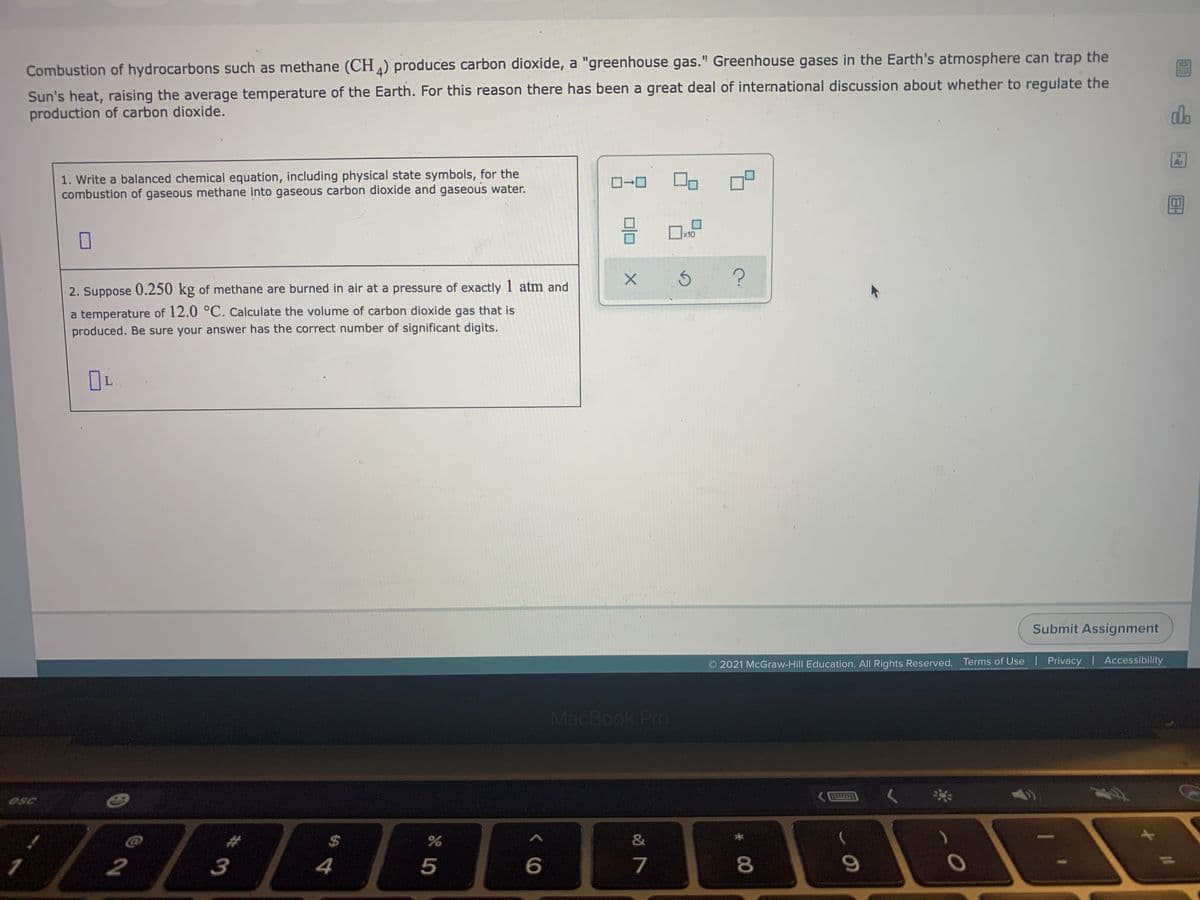
Transcribed Image Text:Combustion of hydrocarbons such as methane (CH ) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the
Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the
production of carbon dioxide.
do
Ar
1. Write a balanced chemical equation, including physical state symbols, for the
combustion of gaseous methane into gaseous carbon dioxide and gaseous water.
x10
2. Suppose 0.250 kg of methane are burned in air at a pressure of exactly 1 atm and
a temperature of 12.0 °C. Calculate the volume of carbon dioxide gas that is
produced. Be sure your answer has the correct number of significant digits.
Submit Assignment
2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Accessibility
MacBook Pro
esc
Tete
$
&
3
5
7
* 00
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co