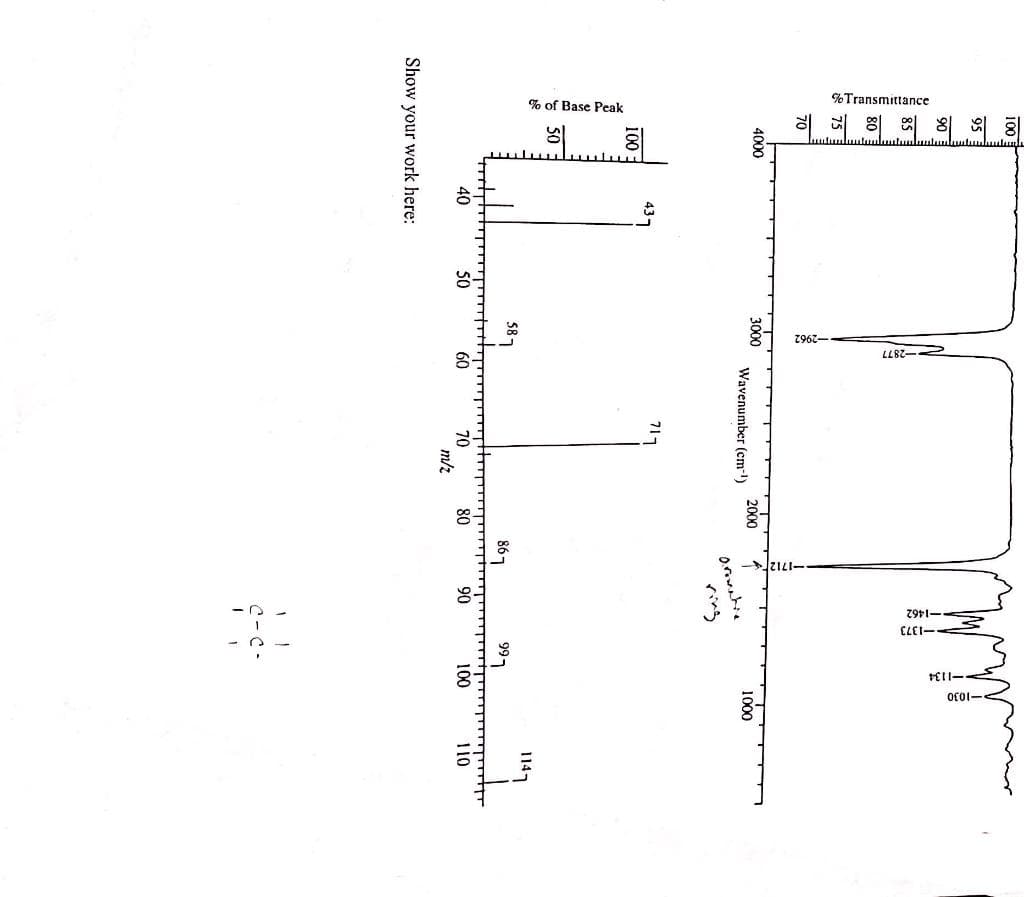
Following are the IR spectrum and mass spectrum of an unknown compound. Propose a structure for the unknown compound. Make sure to explain both IR and mass spectra for showing how you got your structure. For the mass spectrum, show the fragmentation mechanism for peaks at m/z 86,71 and 43.
Following are the IR spectrum and mass spectrum of an unknown compound. Propose a structure for the unknown compound. Make sure to explain both IR and mass spectra for showing how you got your structure. For the mass spectrum, show the fragmentation mechanism for peaks at m/z 86,71 and 43.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter8: An Introduction To Optical Atomic Spectrometry
Section: Chapter Questions
Problem 8.2QAP
Related questions
Question
Following are the IR spectrum and mass spectrum of an unknown compound. Propose a structure for the unknown compound. Make sure to explain both IR and mass spectra for showing how you got your structure. For the mass spectrum, show the fragmentation mechanism for peaks at m/z 86,71 and 43.
Transcribed Image Text:% Transmittance
100
05
4000
3000
Show your work here:
Wavenumber (cm-')
기ㄱ
60
70
2000
m/z
-1462
aromatie
-1373
437
100
고...
1147
587
867
997
80
90
100
110
40
50
--1134
C-C-
>-1030
1000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning