For 223FT, number of protons = number of neutrons = + and number of electrons = For 227 Fr, number of protons = number of neutrons% D • and number of electrons =
For 223FT, number of protons = number of neutrons = + and number of electrons = For 227 Fr, number of protons = number of neutrons% D • and number of electrons =
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.19QAP
Related questions
Question
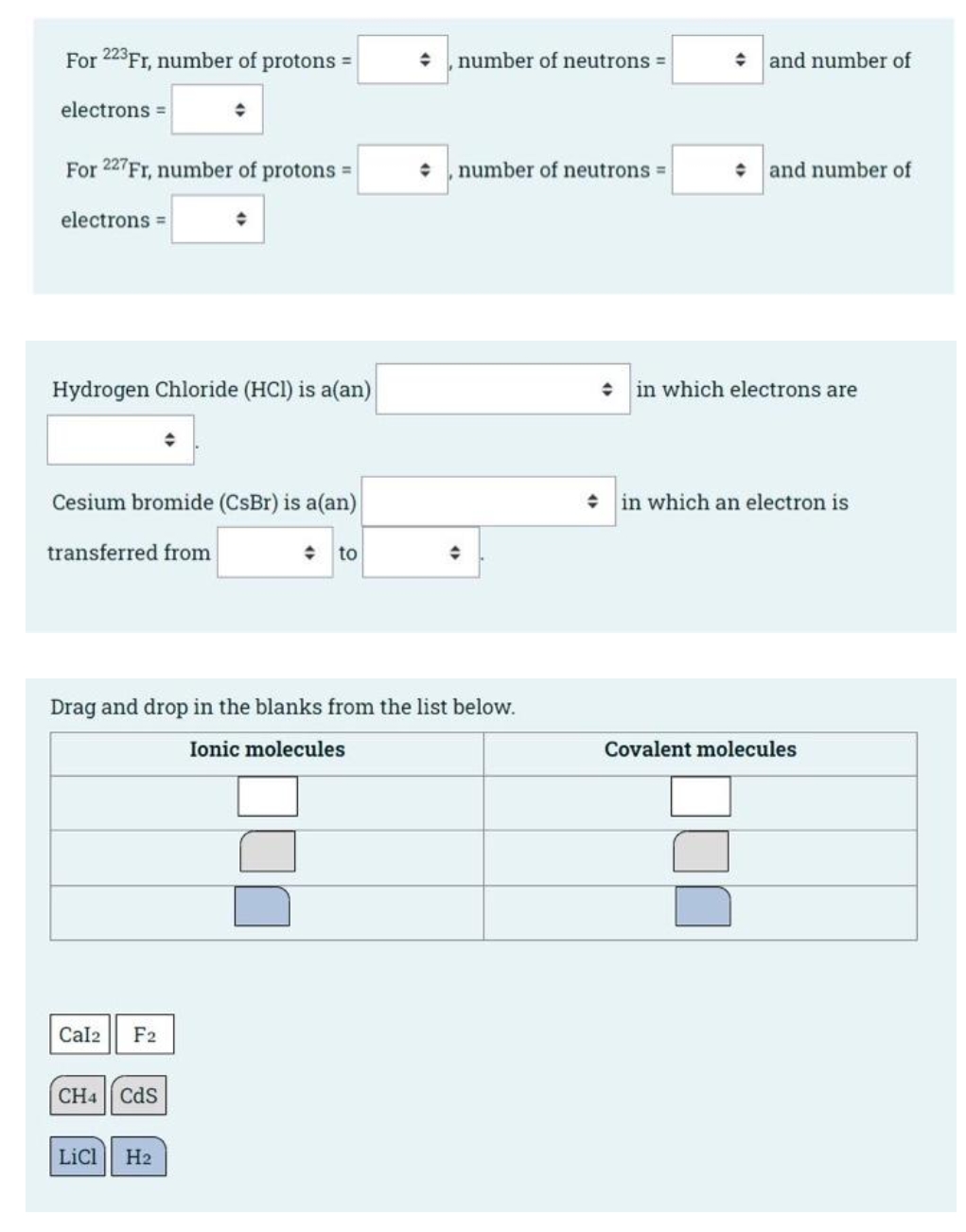
Transcribed Image Text:For 223FT, number of protons =
number of neutrons =
+ and number of
electrons
For 227FT, number of protons =
number of neutrons
• and number of
electrons =
Hydrogen Chloride (HCl) is a(an)
in which electrons are
Cesium bromide (CsBr) is a(an)
+ in which an electron is
transferred from
to
Drag and drop in the blanks from the list below.
Ionic molecules
Covalent molecules
Cal2
F2
CH4 Cds
LiCl
H2
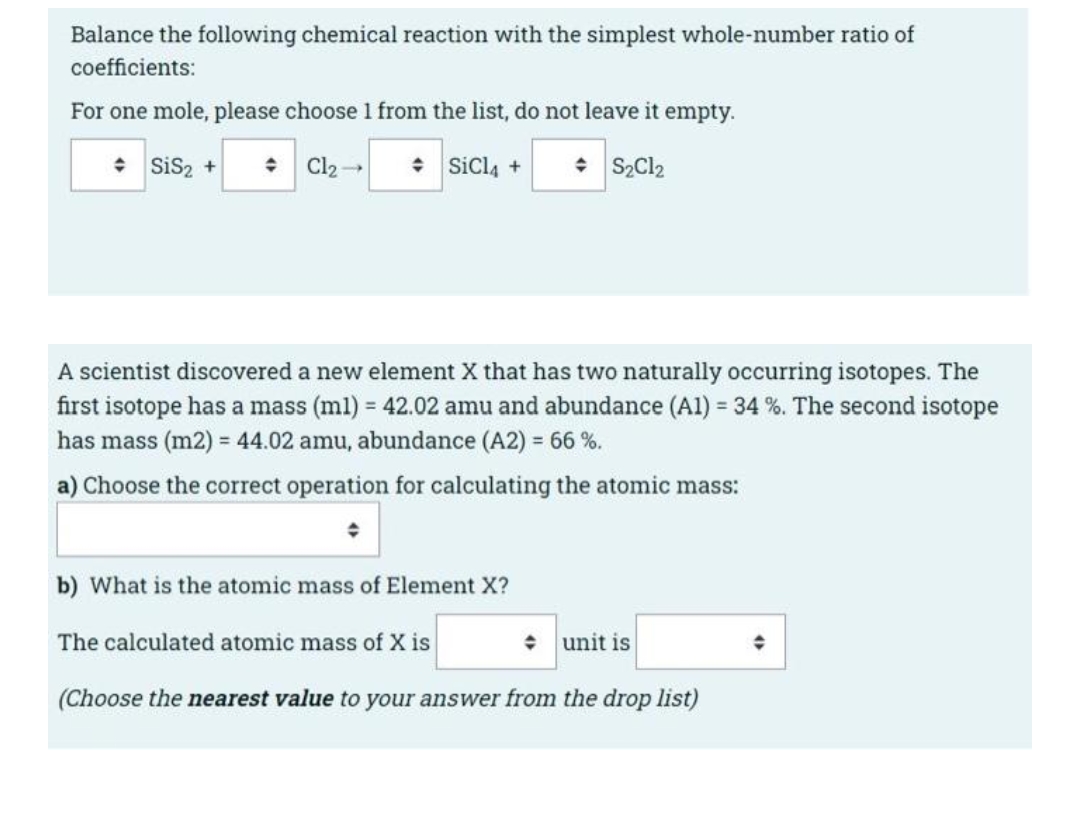
Transcribed Image Text:Balance the following chemical reaction with the simplest whole-number ratio of
coefficients:
For one mole, please choose 1 from the list, do not leave it empty.
* Sis2 +
+ Cl2-
* Sicl4 +
• S2C12
A scientist discovered a new element X that has two naturally occurring isotopes. The
first isotope has a mass (ml) = 42.02 amu and abundance (Al) = 34 %. The second isotope
has mass (m2) = 44.02 amu, abundance (A2) = 66 %.
a) Choose the correct operation for calculating the atomic mass:
b) What is the atomic mass of Element X?
The calculated atomic mass of X is
unit is
(Choose the nearest value to your answer from the drop list)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning