For each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. compound hydrogen-bonding force Between Between molecules of the compound and molecules of water? formula or Lewis name molecules of the structure compound? hypochlorous acid HCIO O yes O yes O no O no O yes O no O yes hydrogen bromide HBr O no H :i: N,N- dichloromethylamine O yes O no O yes H C N- CI: O no
For each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. compound hydrogen-bonding force Between Between molecules of the compound and molecules of water? formula or Lewis name molecules of the structure compound? hypochlorous acid HCIO O yes O yes O no O no O yes O no O yes hydrogen bromide HBr O no H :i: N,N- dichloromethylamine O yes O no O yes H C N- CI: O no
Chapter4: Forces Between Particles
Section: Chapter Questions
Problem 4.107E
Related questions
Question
Do 2 and 3
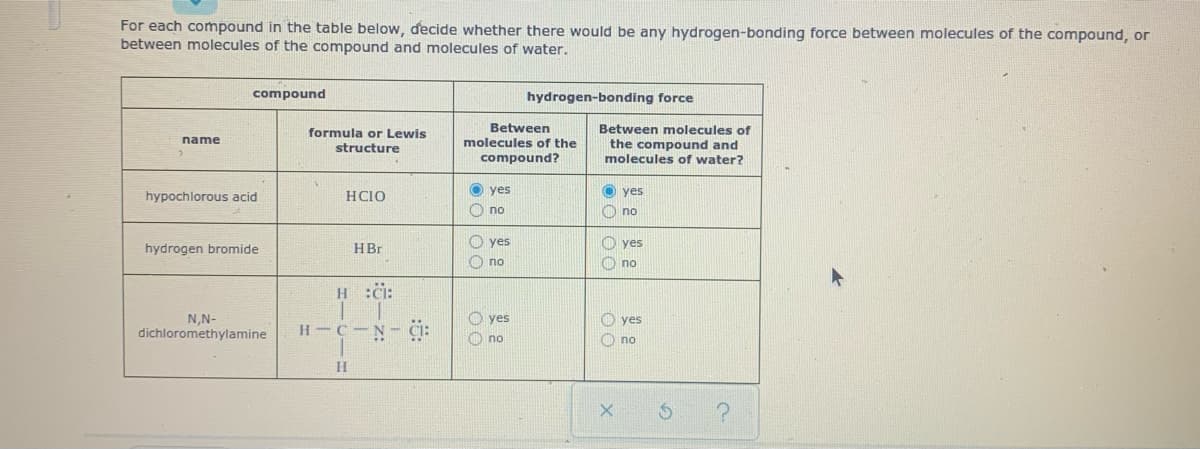
Transcribed Image Text:For each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or
between molecules of the compound and molecules of water.
compound
hydrogen-bonding force
Between
Between molecules of
formula or Lewis
structure
name
molecules of the
the compound and
molecules of water?
compound?
hypochlorous acid
HCIO
O yes
O yes
O no
O no
O yes
O yes
hydrogen bromide
HBr
O no
O no
H :i:
N,N-
dichloromethylamine
O yes
O no
O yes
H C N- CI:
O no
Expert Solution
Step 1
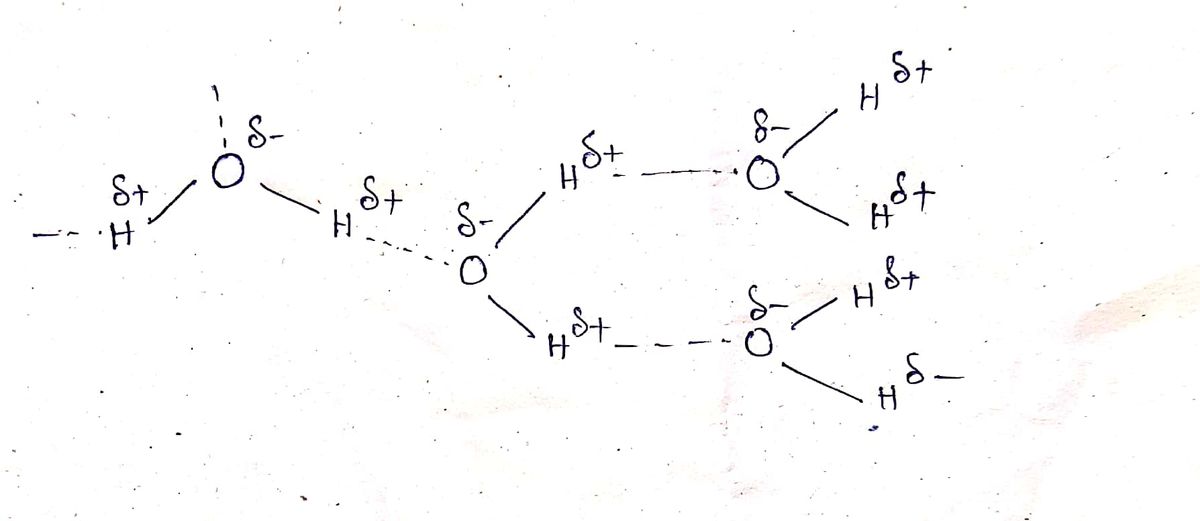
H bonding occurs in those molecules where H is attached with sufficiently electronegative atom so that a polarity is arrises. This acts as H bond donor where as there is another atom that should be sufficiently electronegative.
H bonding in water as follows
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
