Q: In a titration experiment, 50mL of HCI was needed to completely neutralize 20mL of 0.25 M NaOH.…
A: Given :- Molarity of NaOH solution = 0.25 M Volume of NaOH solution = 20 mL Volume of HCl solution =…
Q: A chemist has a solution that has a pH of 3. She adds a chemical to it, and shortly afterwards the…
A: Initial pH of the solution is 3 Final pH of the solution is 5 To determine the concentration of the…
Q: How many milliliters of 0.250 M HNO3 are needed to titrate 35.0 mL of a 0.888 M NaOH solution to its…
A:
Q: You are given three unknown solutions. One is strongly acidic (pH below 1), one is neutral, and one…
A: pH indicators are added in small amount to solutions so that they can indicate their pH by showing…
Q: At one point during the titration, there are 15.0 mL of the 1.0 M LIOH. How many moles OH- are in…
A:
Q: In a titration experiment, it takes 31.45 mL of 0.018 M NaOH to neutralize a 15.00 mL sample of HCI.…
A: Acid reacts with base to form salt and water. In the given reaction acid is HCl. It reacts with NaOH…
Q: How many mL of 0.300 M HCI would be needed to just neutralize 20.0 mL of 0.250 M NaOH?
A:
Q: If 19.5 mL of 0.1 M NaOH is required for the titration of 0.5 M HCl, how many mL of acid is present?
A: Acid-Base Titration: One mole of sodium hydroxide base neutralizes one mole of hydrochloric acid…
Q: If you did not rinse off the pH probe between measuring the pH of the HCl solution and the NaOH…
A: Since we have not rinse off the pH probe after measuring the pH of HCl. Hence it will have some HCl…
Q: mL
A:
Q: A chemist combines 7.0 mL of 3.0 M HCI and 7.0 mL of 3.0 M NaOH. Is the resulting solution acidic,…
A:
Q: A student forgot to label two bottles of acid. One contained HCl and the other HClO. What test could…
A: the answer is B) simply measure and compare the pH of the two and A) measure the pH before and after…
Q: A swimming pool has a volume of one million liters. How many grams of HCI would need to be added to…
A: The question is based on the concept of solutions. we need to determine how much hydrochloric acid…
Q: A chemist combines 7.8 ml of 3.0 M HCI and 6.2 ml of 3.0 M NAOH. Is the resulting solution acidic,…
A: The given acid HCl and base NaOH are both strong in nature, therefore, they would undergo…
Q: True or False: At the equivalence point in a titration, the # moles acid = # moles base.
A: the point at which enough acid and base to neutralize the solution called as equivalence point .
Q: What volume of 0.717 M HCI is required to titrate 11.9 mL of 0.151 M KOH?
A: Chemical equation for the given reaction is: HCl + KOH →KCl + H2O We can use the molarity equation:…
Q: A particular acid-base indicator has an acid ionization constant, KIn= 8.3E-11. What would be the…
A: In the acid base titration, the pKIn signifies the mid-point of the pH range in which a particular…
Q: Acid-base Titration Assignment solves questions in end. if necessary use notebook to make the…
A: Given data: Volume of NaOH added (mL) pH 0.0 2.87 10.0 4.14 25.0 4.74 40.0 5.35 50.0…
Q: v much 0.150M HCI wa
A: At Neutralization point, Gram eq. of LiOH = Gram eq. of HCl Mole×valence factor = N2V2
Q: a) Calculate the volume of NaOH needed to reach the equivalence point.
A:
Q: Acidosis is a human body disorder caused by too much acid in the stomach. If a patient was tested…
A: Given: The patient is tested for acidosis.
Q: How many mls of 0.125 M HCl must be added to 65.5 mls of 0.234 M NH3 to completely neutralize the…
A: Given, Molarity of HCl solution = 0.125 M = 0.125 mol/L Volume of NH3 solution = 65.5 mL = 0.0655 L…
Q: That is an indicator? Give an example and explain how it helps to identify acidic and asic…
A: An Indicator is a substance that changes color or gives a visible sign on changing the pH. For e.g.…
Q: Which graph best represents the titration of ammonia with hydrochloric acid?
A: Ammonia is a weak base and HCl is a strong acid . Titration of Ammonia with HCl is explained with…
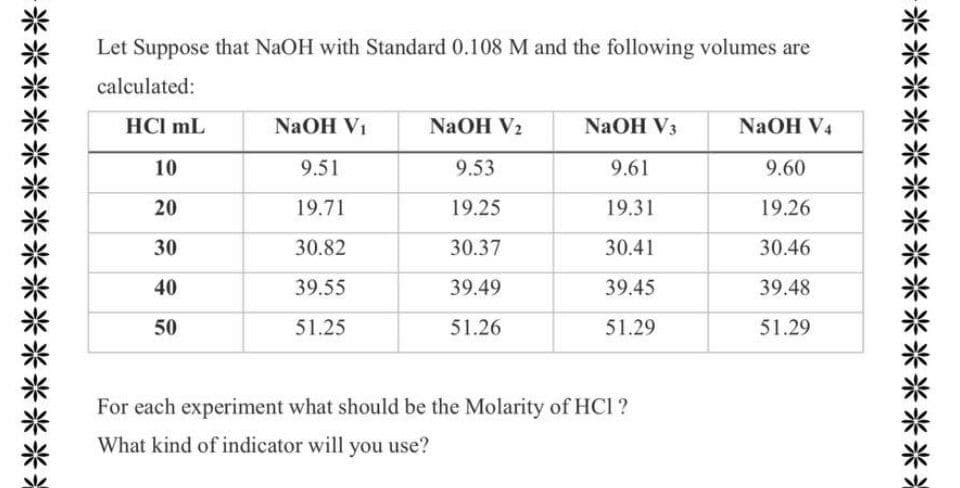
Q: The following items are potential problems someone might encounter while performing a titration.…
A: Forgetting to rinse the burette with NaOH solution causes considerable effect on calculated…
Q: Give examples from the table below that would be best acid base indicator to use for the following…
A: ( By the given table ) Strong acid , strong base – methyl violet , alizarin yellow Weak acid,…
Q: 4. What volume of 0.85 M NaOH would be required to titrate 100 ml of 0.50 M HC1?
A: We have given that Volume of HCl =V1 = 100ml Molarity of HCl=(M1)=0.50M Molarity of NaOH (M2)…
Q: 1. Describe how the technique of back titration could be used in an acid-base neutralization if the…
A: "Since there are multiple question, and it is not specified which question has to solve , i am…
Q: HCI
A:
Q: In this experiment which is the acid and which is the base: 25.00 ml of 0.500 M HCl neutralized…
A:
Q: what we can learn from acid base indicators?
A: Acid - base indicators (also known as pH indicators) are substances which change color with pH. They…
Q: acid-base titration
A: Titration of acid with called acid base titration Or neutralisation . In which a primary standard…
Q: Explain why an acid-base indicator changes color over a range of pH values rather than at a specific…
A:
Q: Which qualitative test does this process involve? NO2 HNO3 H2SO4
A: Nitration is a chemical process of introducing a nitro group into an organic compound.
Q: How many moles of HCI: In a titration experiment, 25.00 ml of 0.500 M HCl neutralized 35.0 ml of…
A: Number of atoms, ions and molecules present on a given amount can be determined by “Mole Concept”.…
Q: What is the effect of dilution on the fraction of the acetic acid ionized?
A: Those chemical compounds that can release protons in the solution are known as the acids. There are…
Q: 1. Prepare a titration curve. Plot pH (on the y-axis) versus volume of NaOH solution added (on the…
A: Titration of HCl solution (strong acid) and NaOH ( strong base) is a neutralization reaction which…
Q: KHP(aq) + NaOH(aq) -> H2O (l) + KNaP(aq) 1. If we forgot to add the phenolphthalein indicator, why…
A: 1. If we forgot to add the phenolphthalein indicator, we will be unable to determine the NaOH…
Q: If we titrate 10.00 mL of a 0.200 M HCl soultion with the sodium hydroxide, we find it requires…
A:
Q: 16 OF 18 QUESTIONS Question 13 In conductometric titration of strong acid with strong base, which…
A: A condutometric titration of strong acid and strong base is ....
Q: The following items are potential problems someone might encounter while performing a titration. For…
A: The molarity of a solution gives the concentration of the solution and is defined as the ratio of…
Q: The following items are potential problems someone might encounter while performing a titration. For…
A: Forgetting to rinse the burette with NaOH solution causes considerable effect on calculated…
Q: Write chemical reaction of HCI with water.
A:
Q: What is the [H3O-] and [OH-] ion concentration respectively in a solution that contains 0.155 M HNO3
A:
Q: How many milliliters of water have to be added to 143.8 ml of 0.25 M HCl to reduce the concentration…
A: Given :- Initial concentration of HCl solution = 0.25 M Initial volume = 143.8 mL Final…
Q: Indicate by a checkmark or X strong electrolyte, weak electrolyte, strong base, weak base, strong…
A: Any aqueous solution which is able to conduct electricity is called as electrolyte. Electrolytes are…
Q: A student is presented with the following data: pH meter reading 10.5 Concentration of H+ ions low…
A: Given details, The pH of the solution = 10.5, The hydrogen ion concentration = low in the solution,…
Q: In a weak acid titration, if the buret was not prepared properly and a considerable amount of water…
A: Weak acid titration:It is the titration of weak acid with strong base, there is proton transfer from…
Q: What does the pKIn of an acid-base indicator mean and how does it help in choosing an appropriate…
A: An indicator depends on its acid strength i.e. pH range where the color of the indicator changes…
Q: What is the endpoint of a titration?
A: In a titration indicators are used for the identification of end point. Equivalence point is the…
Step by step
Solved in 7 steps with 12 images

- The following evidence was obtained from an experiment to determine the solubility of calcium chloride at room temperature. A sample of saturated calcium chloride solution was evaporated to dryness, and the mass of solid residue was measured.EvidenceVolume of solution (mL) = 15.0Mass of empty beaker (g) = 90.54Mass of beaker and residue (g) = 101.36The solubility of calcium chloride is g/100 mLKMnO4 and Na2C2O4 solutions were used in the reactions that took place in a back titration to determine the amount of H2O2 in a sample. Calculate the concentration of H2O2 in the sample (w / v) as% by making appropriate assumptions for the volumes and normality of all these solutions.Pure potassium hydrogen phthalate is used for the standardization of the sodium hydroxide solution. Suppose that the potassium hydrogen phthalate is not completely dry. Will the reported molar concentration of the sodium hydroxide solution be too high, too low, or unaffected because of the moistness of the potassium hydrogen phthalate? Explain.
- In Heat of solution from Solubility experiment: the slope of plot of Ln Sversus 1/T (K) = - 534 and the intercept = 2.964, calculate the solubility SDetermine the effect of Column A to Column B (increase, decrease, or no effect).By the use of Henderson Hasselbalch equation; pH = pKa + log{[acetate ion]/[acetic acid]} 4.5 = 4.75 + log{[0.10 M]/[acetic acid]} -0.25 = log{[0.10 M]/[acetic acid]} [Acetic acid] = 0.10 M/ 10-0.25 [Acetic acid] = 0.10 M/0.56 [Acetic acid] = 0.1786 M Moles of sodium acetate dissolved in 250 mL buffer solution = 0.10 M× (250mL/1000mL) × 1L = 0.025 mol Weight (w) of sodium acetate (purity 100%) dissolved to prepare 250 mL of solution with buffer concentration of 0.10 M is calculate as follow; w100% = 0.025 mol × 82.0343 g/mol = 2.051 g Weight (w) of sodium acetate (purity 99%) is calculate as follow; w99% = 2.051 g× (100/99) = 2.072 g What was the volume of 6.12 M acetic acid HC2H3O2 needed to prepare the 250 mL acetic acid/acetate ion buffer solution required in this part? Show your calculations.
- Estimate the temperature at which the equilibrium constant for CuSO4 ⋅ 5 H2O(s) → CuSO4(s) + 5 H2O(g) becomes 1; assume pH2O = 1 bar.A solution is prepared by dissolving 40.00 g of NaCl (f.w. = 58.44 g mol–1), a non-volatile solute, in enough water (m.w. = 18.02 g mol–1) to result in exactly 1 L of solution at 25 °C. Assume the density of the solution is that of pure water (dsolution = 1.000 g mL–1). The ebullioscopic constant (Kb) for water is 0.513 °C m–1. The cryoscopic constant (Kf) for water is 1.86 °C m–1. The vapor pressure of pure water is 0.0313 atm. Find the freezing point of the solution(in C to 2 decimal places)A solution is prepared by dissolving 40.00 g of NaCl (f.w. = 58.44 g mol–1), a non-volatile solute, in enough water (m.w. = 18.02 g mol–1) to result in exactly 1 L of solution at 25 °C. Assume the density of the solution is that of pure water (dsolution = 1.000 g mL–1). The ebullioscopic constant (Kb) for water is 0.513 °C m–1. The cryoscopic constant (Kf) for water is 1.86 °C m–1. The vapor pressure of pure water is 0.0313 atm. Find the vapor pressure of the solution to 3 decimal places in atm.
- A solution is prepared by dissolving 40.00 g of NaCl (f.w. = 58.44 g mol–1), a non-volatile solute, in enough water (m.w. = 18.02 g mol–1) to result in exactly 1 L of solution at 25 °C. Assume the density of the solution is that of pure water (dsolution = 1.000 g mL–1). The ebullioscopic constant (Kb) for water is 0.513 °C m–1. The cryoscopic constant (Kf) for water is 1.86 °C m–1. The vapor pressure of pure water is 0.0313 atm. Find the osmotic pressure in atm to three decimal placesA solution is prepared by dissolving 40.00 g of NaCl (f.w. = 58.44 g mol–1), a non-volatile solute, in enough water (m.w. = 18.02 g mol–1) to result in exactly 1 L of solution at 25 °C. Assume the density of the solution is that of pure water (dsolution = 1.000 g mL–1). The ebullioscopic constant (Kb) for water is 0.513 °C m–1. The cryoscopic constant (Kf) for water is 1.86 °C m–1. The vapor pressure of pure water is 0.0313 atm. Determine the boiling point of the solution(in C to 2 decimal places)A solution is prepared by dissolving 40.00 g of NaCl (f.w. = 58.44 g mol–1), a non-volatile solute, in enough water (m.w. = 18.02 g mol–1) to result in exactly 1 L of solution at 25 °C. Assume the density of the solution is that of pure water (dsolution = 1.000 g mL–1). The ebullioscopic constant (Kb) for water is 0.513 °C m–1. The cryoscopic constant (Kf) for water is 1.86 °C m–1. The vapor pressure of pure water is 0.0313 atm. Determine the following: Boiling point of solution (in °C to two decimal places) Freezing point of solution (in °C to two decimal places) Vapor pressure of the solution (in atm to three decimal places) Osmotic pressure (in atm to three decimal places)

