Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter14: Applications Of Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 14.16QAP
Related questions
Question
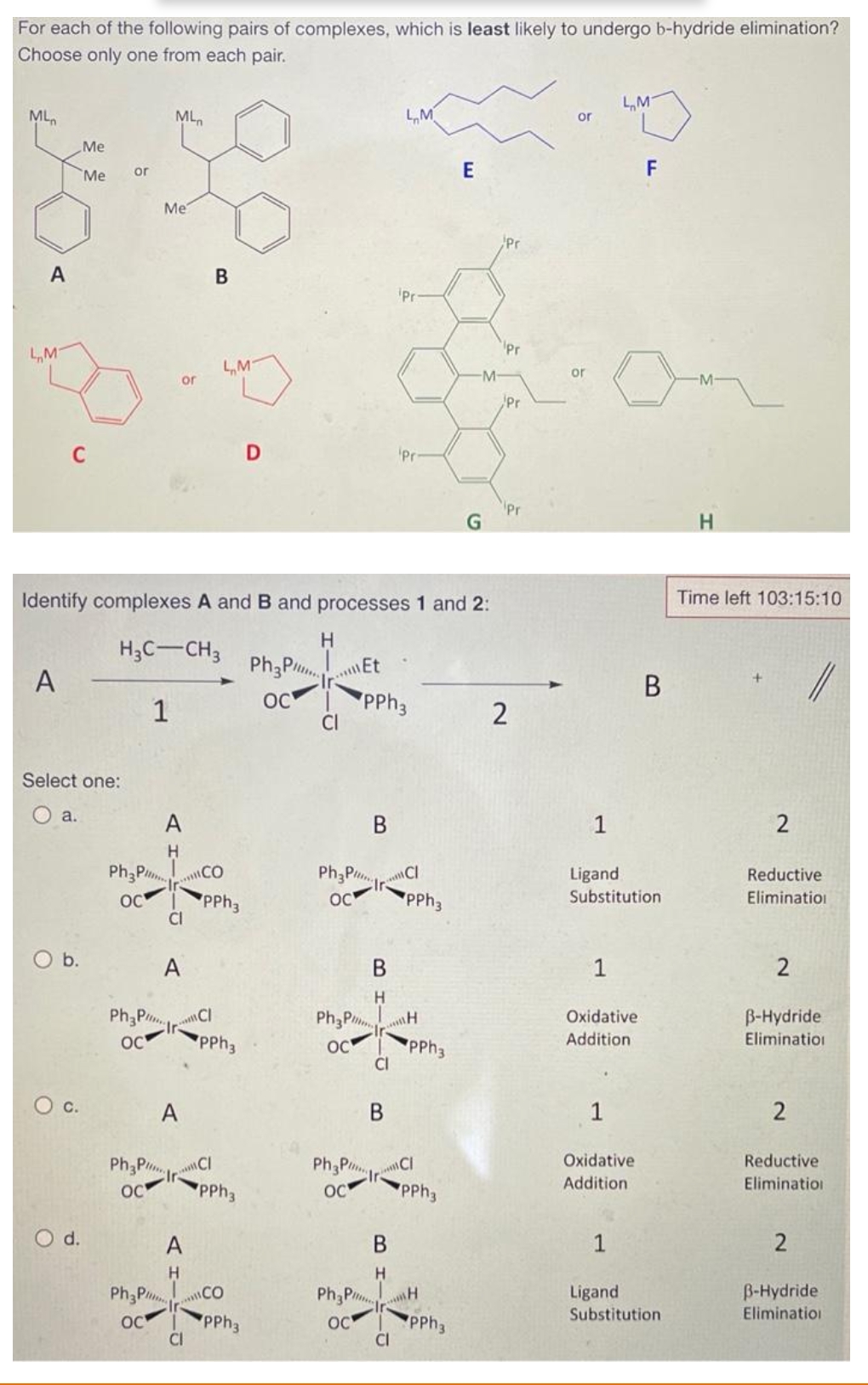
Transcribed Image Text:For each of the following pairs of complexes, which is least likely to undergo b-hydride elimination?
Choose only one from each pair.
ML
A
LMT
A
C
Select one:
a.
Me
Me
O b.
O C.
O d.
or
Ph3P
ос
ML
Me
or
Ph3P
OC
Identify complexes A and B and processes 1 and 2:
H3C-CH3
1
A
H
CI
B
A
Ph3PCI
OC
A
LM
A HIKIC
CO
PPh3
CI
Ph3P Ir-
PPh3
OC
PPh3
D
CO
PPh3
Ph3P
OC
H
.. Et
Ph3P
OC
PhaPr
OC
Pha
B
OC
PPh 3
BH
CI
PhaPr
OC
B
Pi...
B
H
LM
Pr
CI
Pri
CI
PPh3
H
PPh3
MCI
PPh3
E
PPh3
-M-
G
Pr
Pr
Pr
2
or
or
LM
1
Ligand
Substitution
Oxidative
Addition
F
Oxidative
Addition
B
1
Ligand
Substitution
H
Time left 103:15:10
Reductive
Elimination
B-Hydride
Elimination
2
Reductive
Elimination
2
B-Hydride
Elimination

Transcribed Image Text:For the reaction of the sodium enolate with chlorotrimethylsilane shown below, what is the
thermodynamic driving force for this reaction?
+ CISiMe3
Me Si-O
NaO
+ NaCl
Select one:
A. The formation of strong covalent bonds between Sodium and Chloride ions.
B. The breaking of weak Na-O and Si-Cl bonds.
C. Silicon-oxygen silicon bonds are stronger than silicon-carbon single bonds.
D. The formation of the NaCl lattice and release of the lattice enthalpy, coupled with the
formation of a strong Si-O bond.
For organolithium clusters of the general formula (RLi)6, reaction with additional Lewis basic
donor ligands leads to deaggregation and formation of smaller rings than the 6 membered
rings seen in the original cluster. For internally solvated clusters, however, 6 membered rings
are possible. Why?
Select one:
O A. Internal solvation does not significantly increase the effective steric demand of the
organic fragment, whilst maximising R-Li contacts.
B. Internal solvation is sterically demanding, which forces larger rings to be adopted.
OC. The organolithium deprotonates the Lewis base, leading to loss of reagent and so
smaller rings.
OD. Internal solvation disrupts the normal solvation sphere, leading to lower coordination
numbers at Li.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

