For each of the following pairs of solutes and solvent, predict whether the solute would be d. Aspirin in water soluble or insoluble. After making your predictions, you can check your answers by looking up the compounds in The Merck Index or the CRC Handbook of Chemistry and Physics. Gen- erally, The Merck Index is the easier reference book to use. If the substance has a solubility greater than 40 mg/mL, you may conclude that it is soluble. a. Malic acid in water С—ОН 0-C- -CH3 Aspirin НО—С CHCH2- С—ОН e. Succinic acid in hexane (Note: The polarity of hexane is similar to that of petroleum ether.) ОН Ma ic acid b. Naphthalene in water || НО-С—СH2CH2—С—ОН 6 cinic acid f. Ibuprofen in diethyl ether Naphthalene CH3 CH3 0 c. Amphetamine in ethyl alcohol CH3CHCH2 · CH—CОН NH2 Ibupr6 en -CH,CHCH3 g. 1-Decanol (n-decyl alcohol) in water Amphetamine CH,(CH,),CH,OH D еcab
For each of the following pairs of solutes and solvent, predict whether the solute would be d. Aspirin in water soluble or insoluble. After making your predictions, you can check your answers by looking up the compounds in The Merck Index or the CRC Handbook of Chemistry and Physics. Gen- erally, The Merck Index is the easier reference book to use. If the substance has a solubility greater than 40 mg/mL, you may conclude that it is soluble. a. Malic acid in water С—ОН 0-C- -CH3 Aspirin НО—С CHCH2- С—ОН e. Succinic acid in hexane (Note: The polarity of hexane is similar to that of petroleum ether.) ОН Ma ic acid b. Naphthalene in water || НО-С—СH2CH2—С—ОН 6 cinic acid f. Ibuprofen in diethyl ether Naphthalene CH3 CH3 0 c. Amphetamine in ethyl alcohol CH3CHCH2 · CH—CОН NH2 Ibupr6 en -CH,CHCH3 g. 1-Decanol (n-decyl alcohol) in water Amphetamine CH,(CH,),CH,OH D еcab
Chapter79: Solubility
Section: Chapter Questions
Problem 1P
Related questions
Question
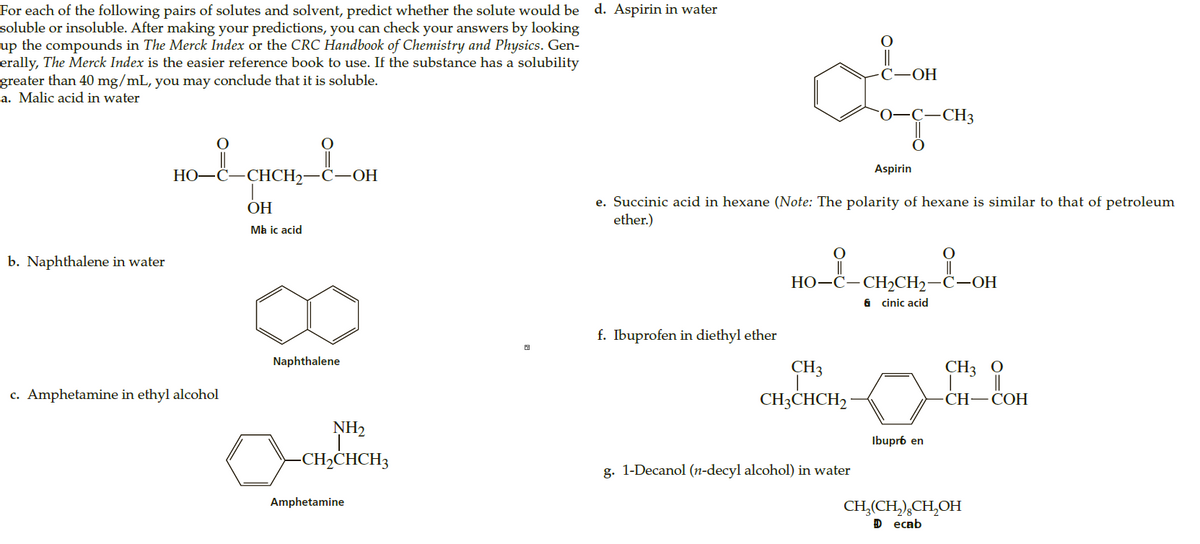
Transcribed Image Text:For each of the following pairs of solutes and solvent, predict whether the solute would be d. Aspirin in water
soluble or insoluble. After making your predictions, you can check your answers by looking
up the compounds in The Merck Index or the CRC Handbook of Chemistry and Physics. Gen-
erally, The Merck Index is the easier reference book to use. If the substance has a solubility
greater than 40 mg/mL, you may conclude that it is soluble.
a. Malic acid in water
С—ОН
0-C-
-CH3
Aspirin
НО—С
CHCH2-
С—ОН
e. Succinic acid in hexane (Note: The polarity of hexane is similar to that of petroleum
ether.)
ОН
Ma ic acid
b. Naphthalene in water
||
НО-С—СH2CH2—С—ОН
6 cinic acid
f. Ibuprofen in diethyl ether
Naphthalene
CH3
CH3 0
c. Amphetamine in ethyl alcohol
CH3CHCH2 ·
CH—CОН
NH2
Ibupr6 en
-CH,CHCH3
g. 1-Decanol (n-decyl alcohol) in water
Amphetamine
CH,(CH,),CH,OH
D еcab
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
