For kerosene : Mass of vial and kerosene : 8.62 g Mass of empty vial 5.80 g Mass of chloroform Mass of kerosene Density of kerosene -- %3D Volume of chloroform
For kerosene : Mass of vial and kerosene : 8.62 g Mass of empty vial 5.80 g Mass of chloroform Mass of kerosene Density of kerosene -- %3D Volume of chloroform
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 13QAP
Related questions
Question
![Act. no. 4 and 5 Measurements and Layering Liquids [Read-Only] - Word
Leila Clarriz Batain
File
Home
Insert
Draw
Design
Layout
References
Mailings
Review
View
Help
O Tell me what you want to do
& Share
X Cut
P Find
12
A A
Aa v
AaBbCcI AaBbCCI AaBbC AaBbCcC AaB AaBbCcDc AaBbCcD
Arial
响Copy
akc Replace
Paste
BIU v abe
A • aly - A-
1 Normal
I No Spac. Heading 1 Heading 2
Subtle Em..
X,
Title
Subtitle
Format Painter
A Select
Clipboard
Font
Paragraph
Styles
Editing
For kerosene :
Mass of vial and kerosene :
8.62 g
Mass of empty vial
5.80 g
Mass of chloroform
Mass of kerosene
Density of kerosene =
Volume of chloroform
Page 3 of 3
551 words
210%
12:55 PM
P Type here to search
Links
31°C
10/29/2021
II](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Febb75db4-2541-4b16-856d-91a47b47f2a2%2Ffeb63dee-b59b-4ab1-a997-585df30d218b%2F9sqbo9e_processed.png&w=3840&q=75)
Transcribed Image Text:Act. no. 4 and 5 Measurements and Layering Liquids [Read-Only] - Word
Leila Clarriz Batain
File
Home
Insert
Draw
Design
Layout
References
Mailings
Review
View
Help
O Tell me what you want to do
& Share
X Cut
P Find
12
A A
Aa v
AaBbCcI AaBbCCI AaBbC AaBbCcC AaB AaBbCcDc AaBbCcD
Arial
响Copy
akc Replace
Paste
BIU v abe
A • aly - A-
1 Normal
I No Spac. Heading 1 Heading 2
Subtle Em..
X,
Title
Subtitle
Format Painter
A Select
Clipboard
Font
Paragraph
Styles
Editing
For kerosene :
Mass of vial and kerosene :
8.62 g
Mass of empty vial
5.80 g
Mass of chloroform
Mass of kerosene
Density of kerosene =
Volume of chloroform
Page 3 of 3
551 words
210%
12:55 PM
P Type here to search
Links
31°C
10/29/2021
II
Transcribed Image Text:chem 103 lab. manual 2021.pdf x
O File C:/Users/user/Downloads/chem%20103%20lab.%20manual%202021.pdf
D Page view A Read aloud
T Add text
V Draw
9 Highlight
O Erase
35
of 97
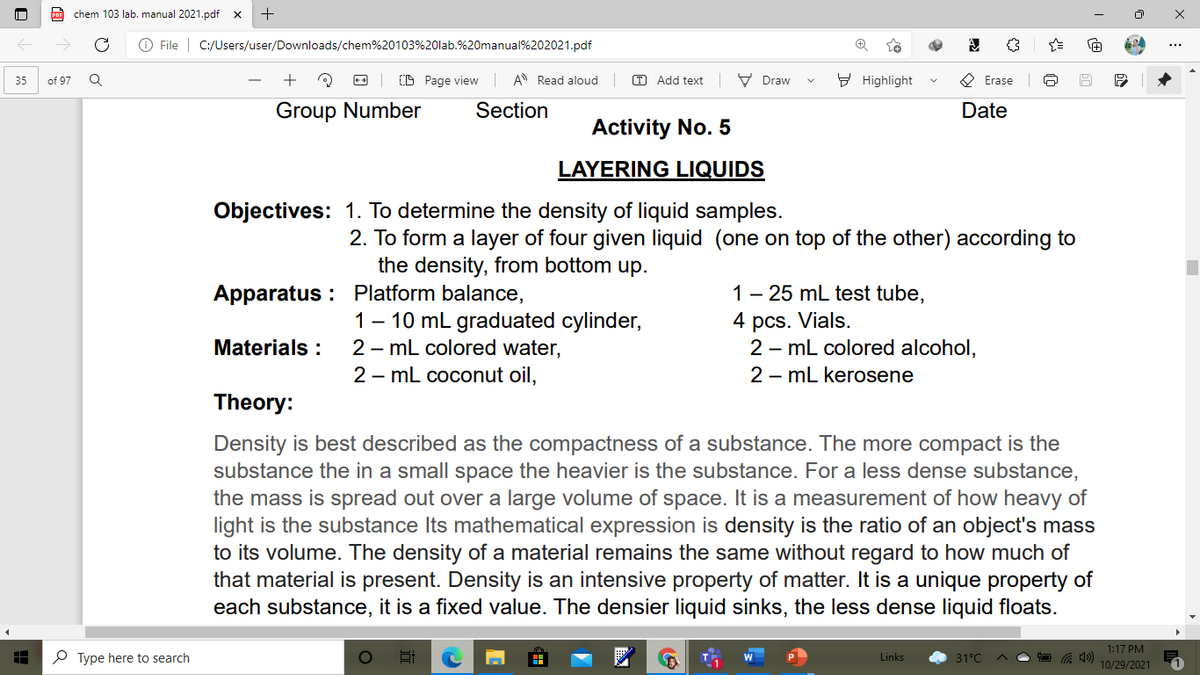
Group Number
Section
Date
Activity No. 5
LAYERING LIQUIDS
Objectives: 1. To determine the density of liquid samples.
2. To form a layer of four given liquid (one on top of the other) according to
the density, from bottom up.
Apparatus : Platform balance,
1 - 25 mL test tube,
1 - 10 mL graduated cylinder,
2 - mL colored water,
2 – mL coconut oil,
4 pcs. Vials.
2 - mL colored alcohol,
2 - mL kerosene
Materials :
Theory:
Density is best described as the compactness of a substance. The more compact is the
substance the in a small space the heavier is the substance. For a less dense substance,
the mass is spread out over a large volume of space. It is a measurement of how heavy of
light is the substance Its mathematical expression is density is the ratio of an object's mass
to its volume. The density of a material remains the same without regard to how much of
that material is present. Density is an intensive property of matter. It is a unique property of
each substance, it is a fixed value. The densier liquid sinks, the less dense liquid floats.
1:17 PM
P Type here to search
Links
31°C
10/29/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning