For the dehydration experiment, the mass of green crystals before heating was G.H g. After the experiment, it was found the mass of water removed was 0.GH g. Calculate the percent water by mass.
For the dehydration experiment, the mass of green crystals before heating was G.H g. After the experiment, it was found the mass of water removed was 0.GH g. Calculate the percent water by mass.
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.60E
Related questions
Question
For #2 the GH is 45 (G.H =4.5)
Mass percent for oxalate is 60% on both trials

Transcribed Image Text:Data Table
Thal #l
Tral #2
Molanty kMn Oq
0. 04M
0.04 m
inital bret volume
4.20ML
I1.70ML
Mass of Green crystal 0.1162 9
U.10559
11.70ML
18.30ML
final buret volume
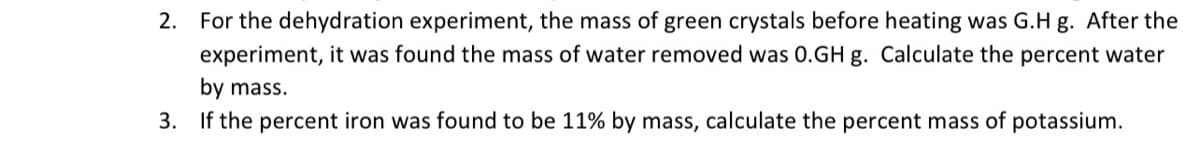
Transcribed Image Text:2. For the dehydration experiment, the mass of green crystals before heating was G.H g. After the
experiment, it was found the mass of water removed was 0.GH g. Calculate the percent water
by mass.
3. If the percent iron was found to be 11% by mass, calculate the percent mass of potassium.
Expert Solution
Step 1
Since you have posted multiple questions, we are entitled to answer the first only
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning