For the gas phase decomposition of dinitrogen pentoxide, N2O52 NO, + 1/2 0, the rate constant has been determined at several temperatures. When In k in s' is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -1.24x104 K and a y-intercept of 31.3. The activation energy for the gas phase decomposition of dinitrogen pentoxide is kJ/mol.
For the gas phase decomposition of dinitrogen pentoxide, N2O52 NO, + 1/2 0, the rate constant has been determined at several temperatures. When In k in s' is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -1.24x104 K and a y-intercept of 31.3. The activation energy for the gas phase decomposition of dinitrogen pentoxide is kJ/mol.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 98QRT: For a reaction involving the decomposition of a hypothetical substance Y, these data are...
Related questions
Question
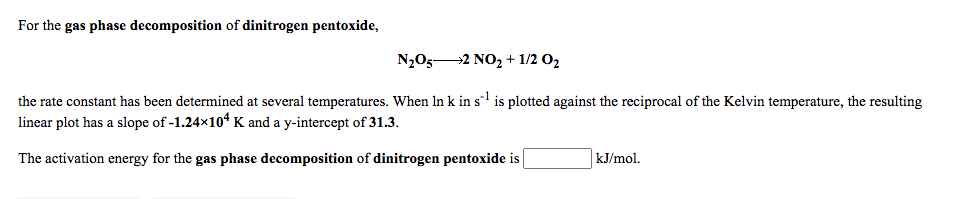
Transcribed Image Text:For the gas phase decomposition of dinitrogen pentoxide,
N2O52 NO, + 1/2 0,
the rate constant has been determined at several temperatures. When In k in s' is plotted against the reciprocal of the Kelvin temperature, the resulting
linear plot has a slope of -1.24x104 K and a y-intercept of 31.3.
The activation energy for the gas phase decomposition of dinitrogen pentoxide is
kJ/mol.
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning