For the reaction A – products, concentration and time data were collected. t (s) [A] (M) Enter these data into the graphing tool to determine the reaction order. 0.0 0.900 25.0 0.609 50.0 0.461 75.0 0.370 Graphing Tool Clear All Data (A) vs. t In[A] vs. t 1/(A) vs. t (A) In[A] 1/[A] 0.9 0.0 0.900 -0.10536 1.111111 25.0 0.609 -0.49594 1.642036 0.7758 50.0 0.461 -0.77436 2.169197 0.6516 75.0 0.370 -0.99425 2.702703 0.5274 0.4032 0.279 15 30 45 60 75 [V)
For the reaction A – products, concentration and time data were collected. t (s) [A] (M) Enter these data into the graphing tool to determine the reaction order. 0.0 0.900 25.0 0.609 50.0 0.461 75.0 0.370 Graphing Tool Clear All Data (A) vs. t In[A] vs. t 1/(A) vs. t (A) In[A] 1/[A] 0.9 0.0 0.900 -0.10536 1.111111 25.0 0.609 -0.49594 1.642036 0.7758 50.0 0.461 -0.77436 2.169197 0.6516 75.0 0.370 -0.99425 2.702703 0.5274 0.4032 0.279 15 30 45 60 75 [V)
Chapter13: Kinetic Methods
Section: Chapter Questions
Problem 9P
Related questions
Question
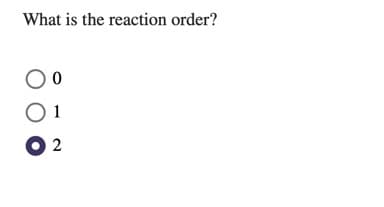
Transcribed Image Text:What is the reaction order?
1
O 2
![For the reaction A → products, concentration and time data were collected.
t (s)
[A] (M)
Enter these data into the graphing tool to determine the reaction order.
0.0
0.900
25.0
0.609
50.0
0.461
75.0
0.370
Graphing Tool
Clear All Data
[A] vs. t
In[A] vs. t
1/[A] vs. t
t
[A]
In[A]
1/[A]
0.9
0.0
0.900
-0.10536
1.111111
25.0
0.609
-0.49594
1.642036
0.7758
50.0
0.461
-0.77436
2.169197
0.6516
75.0
0.370
-0.99425
2.702703
0.5274
0.4032
0.279
15
30
45
60
75
- 8
[V]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F5a207364-abe5-457b-ac18-b934c4bc22d8%2F8f78336c-04b4-4ff3-b7fb-2f1eb8474a8e%2Fdjzgub_processed.jpeg&w=3840&q=75)
Transcribed Image Text:For the reaction A → products, concentration and time data were collected.
t (s)
[A] (M)
Enter these data into the graphing tool to determine the reaction order.
0.0
0.900
25.0
0.609
50.0
0.461
75.0
0.370
Graphing Tool
Clear All Data
[A] vs. t
In[A] vs. t
1/[A] vs. t
t
[A]
In[A]
1/[A]
0.9
0.0
0.900
-0.10536
1.111111
25.0
0.609
-0.49594
1.642036
0.7758
50.0
0.461
-0.77436
2.169197
0.6516
75.0
0.370
-0.99425
2.702703
0.5274
0.4032
0.279
15
30
45
60
75
- 8
[V]
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you



Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning



Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
