For the wave of light you generated in the Part B, calculate the amaunt of energy in 1.0 mol of pnotons with that same frequenty (9.4x10° Hz ) and wavelength (0 032 m) Recall that the Avogadro constant is 6.022 x 10 mol- Express the energy in joules to two significant figures.
For the wave of light you generated in the Part B, calculate the amaunt of energy in 1.0 mol of pnotons with that same frequenty (9.4x10° Hz ) and wavelength (0 032 m) Recall that the Avogadro constant is 6.022 x 10 mol- Express the energy in joules to two significant figures.
Chapter7: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 35Q: Does the minimization of electron-electron repulsions correlate with Hund's rule?
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
100%
Transcribed Image Text:三
CHM 1045
michael
<Post Lecture Homework Chapter 07
PHET Simulation - Wave on a String
2 of 9
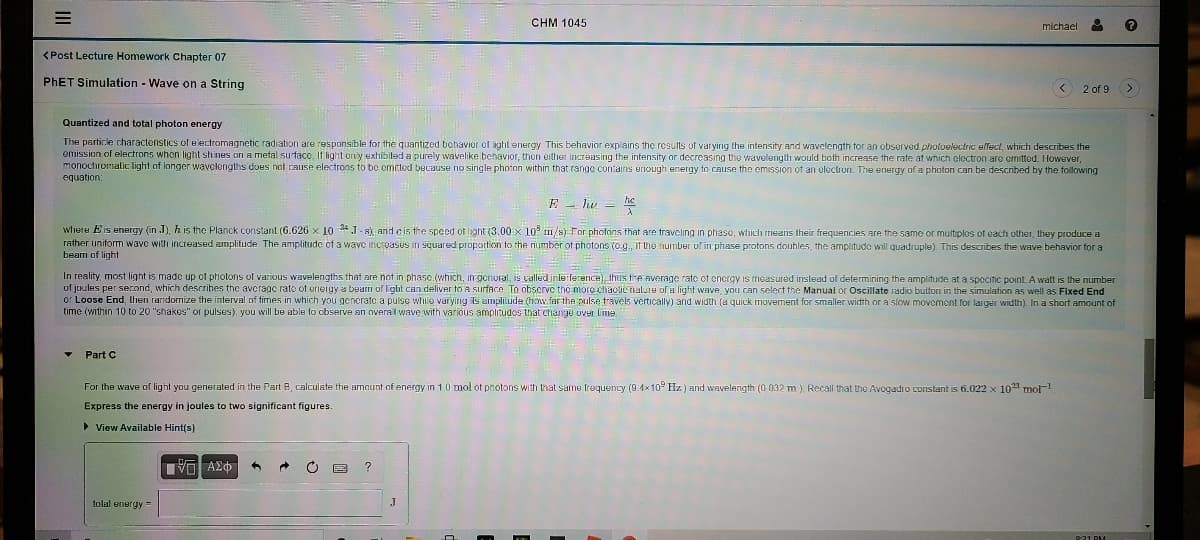
Quantized and total photon energy
The particle charactoristics of e lectromagnetic radiation are responsible for the quantized bchavior of ight energy This behavior explains the results of varying the intensity and wavelength tor an observed photoelectric ulfect, which describes the
emission of electrons whon light shines on a metal surfacc, If light only exhibited a purely wavelike behavior, then oither increasing the intensity or decreasing the wavelength would both increase the rate at which electron aro omitted. However,
monochromatic light of longer wavclengths does net cause electrons to be omrtod because no single photon within that range contains enough energy to cause the emission of an electron. The energy of a photon can be described by the following
equation:
E - hv = e
where Eis energy (in J), h is the Planck constant (6.626 x 10 4J. s), and cis the speed of light (3.00 x 10 1/s) For phofons that are travcling in phase, which means their frequencies are the same or multiplos of oach other, they produce a
rather unitorm wave with increased amplitude The amplitude cf a waye incroasus in squared proportion to the number of photons (e.g., if the number uf in phase protons douhles, the amplitudo will quadruple). This describes the wave behavior for a
beam of light
In reality, most light is made up of photons of various wavelengths that are not in phase (which, In genoral, is called inlerference), thus tre Average rate of energy is measured inslead of determining the amplitude at a speciie point. A watt is the number
of joules per second, which describes the avcrage rate of erneigy a beam of light can deliver to a surface. To obscrvc the more chactic nalure of a light wave, you can select the Manual or Oscillate iadio button in the simulation as well as Fixed End
or Loose End, lhen randorrize the interval of times in which you gencratc a pulse while varying is amplitude (how far the pulse travels vertically) and width (a quick movement for smaller width or a slow movement for larger width) In a short amount of
time (within 10 to 20 "shakes" or pulses), you will be able to cbserve an overal wave with various amplitudos that change UVer lime.
Part C
For the wave of light you generated in the Part B, calculate the amount of energy in 1.0 mol of photons with that same frequenty (9.4x10° Hz ) and wavelength (0 032 m ) Recall that the Avogadro constant is 6.022 x 10 mo-1
Express the energy in joules to two significant figures.
> View Available Hint(s)
tolal energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning