In this activity you will use the virtual lab to determine the concentration of a strong monoprotic acid. To do this, you can perform a titration using NaOH and phenolphthalein found in the virtual lab. (Note: The concentration of the acid is between 0.025M and 2.5M so you will need to dilute the NaOH solution so that the volume to reach the endpoint is between 10 and 50 mL). Once you have determined the concentration of the acid, please enter your answer into a form at the bottom of this page.
In this activity you will use the virtual lab to determine the concentration of a strong monoprotic acid. To do this, you can perform a titration using NaOH and phenolphthalein found in the virtual lab. (Note: The concentration of the acid is between 0.025M and 2.5M so you will need to dilute the NaOH solution so that the volume to reach the endpoint is between 10 and 50 mL). Once you have determined the concentration of the acid, please enter your answer into a form at the bottom of this page.
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 10P
Related questions
Question

Transcribed Image Text:Determine the Concentration of the Unknown Strong Acid
In this activity you will use the virtual lab to determine the concentration of a strong monoprotic acid. To do this, you can perform a titration using
NaOH and phenolphthalein found in the virtual lab. (Note: The concentration of the acid is between 0.025M and 2.5M so you will need to dilute the
NaOH solution so that the volume to reach the endpoint is between 10 and 50 mL).
Once you have determined the concentration of the acid, please enter your answer into a form at the bottom of this page.
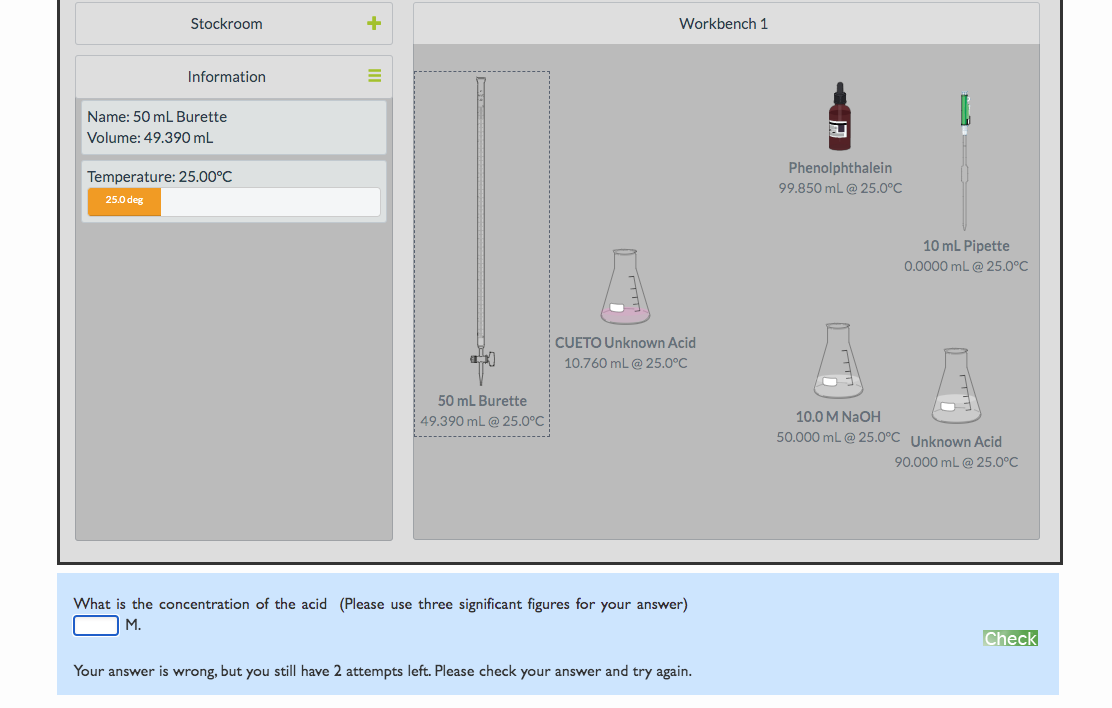
Transcribed Image Text:Stockroom
Workbench 1
Information
Name: 50 mL Burette
Volume: 49.390 mL
Phenolphthalein
Temperature: 25.00°C
99.850 mL @ 25.0°C
25.0 deg
10 mL Pipette
0.0000 mL @ 25.0°C
CUETO Unknown Acid
10.760 mL @ 25.0°C
50 mL Burette
| 49.390 mL @ 25.0°C
10.0 M NAOH
50.000 mL @ 25.0°C Unknown Acid
90.000 mL @ 25.0°C
What is the concentration of the acid (Please use three significant figures for your answer)
M.
Check
Your answer is wrong, but you still have 2 attempts left. Please check your answer and try again.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
For this virtual lab you will have to determine the concentration of a strong monoprotic acid. You will first have to dilute the 10 M sodium hydroxide solution, as your acid will have a concentration between 0.25 M and 2.5 M. You have to use between 10 mL and 50 mL of your diluted sodium hydroxide solution during the titration.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co



Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning