For those anions that usually form soluble compounds, which cations result in the formation of insoluble compounds? List each cation separately.
For those anions that usually form soluble compounds, which cations result in the formation of insoluble compounds? List each cation separately.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 52PS: The dispersed phase of a certain colloidal dispersion consists of spheres of diameter 1.0 102 nm....
Related questions
Question
For those anions that usually form soluble compounds, which cations result in the formation of insoluble compounds? List each cation separately.
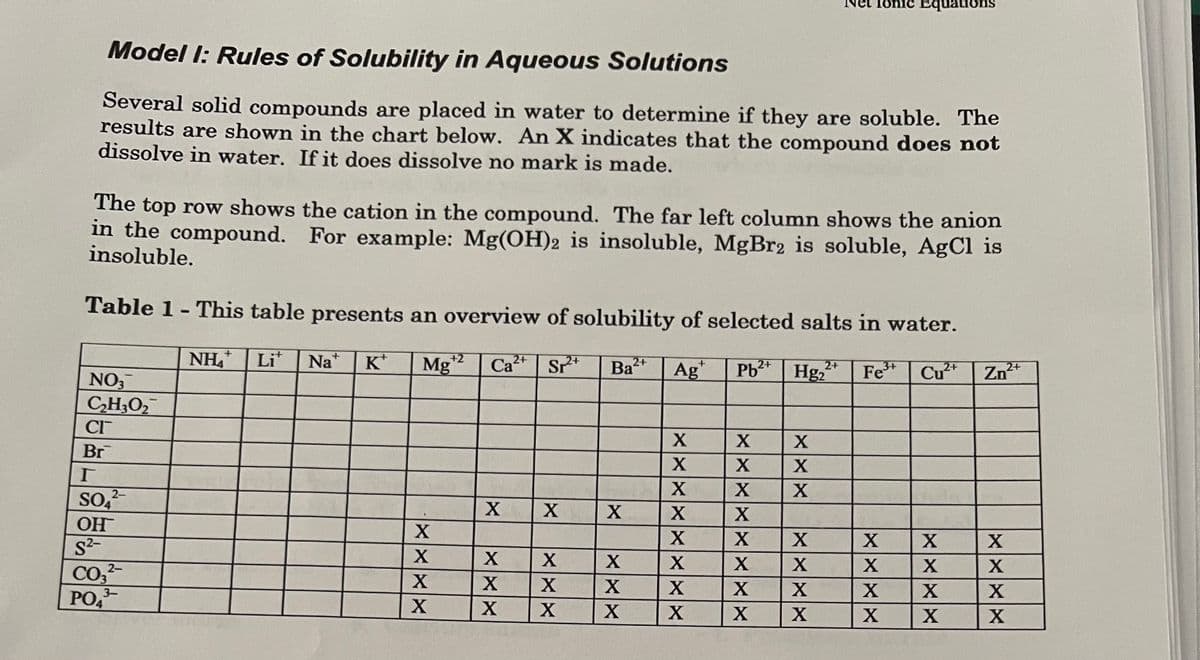
Transcribed Image Text:Model I: Rules of Solubility in Aqueous Solutions
Several solid compounds are placed in water to determine if they are soluble. The
results are shown in the chart below. An X indicates that the compound does not
dissolve in water. If it does dissolve no mark is made.
The top row shows the cation in the compound. The far left column shows the anion
in the compound. For example: Mg(OH)2 is insoluble, MgBr2 is soluble, AgCl is
insoluble.
Table 1- This table presents an overview of solubility of selected salts in water.
+2
NH Lit Na
2+
Mg¹2 Ca2+ Sr²+ Ba2+
2+
Pb²+
Hg₂ Fe3+ Cu²+
NO3
C₂H3O₂
CI
Br
Г
SO4
OH
S²-
2-
2-
CO3
PO43
+
K*
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
Ag
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
Equations
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
2+
Zn
X
X
X
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning