Formation of carbon dioxide Data: Standard molar enthalpy of formation of CO2 (g) at 300 K: AH°(CO2, g; 300 K) = - 393.09 kJ mol- Molar heat capacities at constant pressure (Cp): C (graphite) 20.27 J mol·' K-' O2 (g) 36.57 J mol·' K-' CO2 (g) 31.56 J mol·' K-' Let's consider the reaction of formation of CO2 (g) and its associated molar enthalpy of formation at 1200 K. - This reaction can be considered as another particular case of reaction. Which one? Calculate the standard molar enthalpy of formation of CO2 (g) at 1200 K.
Formation of carbon dioxide Data: Standard molar enthalpy of formation of CO2 (g) at 300 K: AH°(CO2, g; 300 K) = - 393.09 kJ mol- Molar heat capacities at constant pressure (Cp): C (graphite) 20.27 J mol·' K-' O2 (g) 36.57 J mol·' K-' CO2 (g) 31.56 J mol·' K-' Let's consider the reaction of formation of CO2 (g) and its associated molar enthalpy of formation at 1200 K. - This reaction can be considered as another particular case of reaction. Which one? Calculate the standard molar enthalpy of formation of CO2 (g) at 1200 K.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.85QE: The octane number of gasoline is based on a comparison of the gasolines behavior with that of...
Related questions
Question
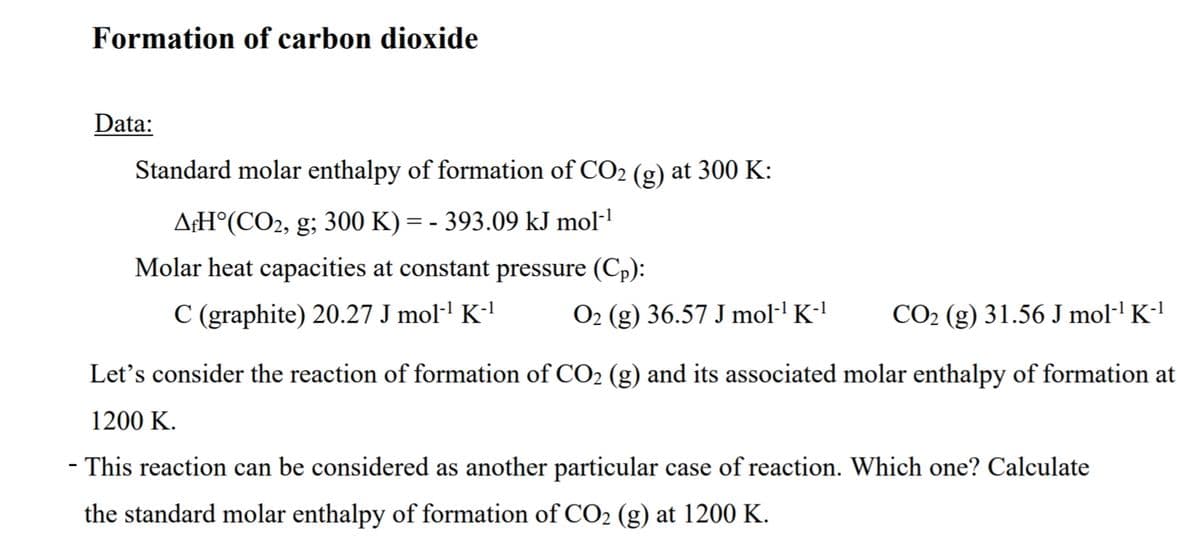
Transcribed Image Text:Formation of carbon dioxide
Data:
Standard molar enthalpy of formation of CO2 (g) at 300 K:
A¢H°(CO2, g; 300 K) = - 393.09 kJ mol-!
Molar heat capacities at constant pressure (Cp):
C (graphite) 20.27 J mol·' K-'
O2 (g) 36.57 J mol·' K-'
CO2 (g) 31.56 J mol·' K-'
Let's consider the reaction of formation of CO2 (g) and its associated molar enthalpy of formation at
1200 K.
- This reaction can be considered as another particular case of reaction. Which one? Calculate
the standard molar enthalpy of formation of CO2 (g) at 1200 K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning