G Gizmos lonic Bonds Select a metal Lithium (LI) Select nonmetal Fluorine (F) Show formula Show completed compounds Hide inner electrons Hint and feedback Drag electrons from metal to nonmetal atoms. Use the Add metal or Add nonmetal buttons to add more atoms. When you are finished, press Check Add metal Add nonmetal Show charge Lithium Fluorine Check Reset Controls: %3D Speed Tools
G Gizmos lonic Bonds Select a metal Lithium (LI) Select nonmetal Fluorine (F) Show formula Show completed compounds Hide inner electrons Hint and feedback Drag electrons from metal to nonmetal atoms. Use the Add metal or Add nonmetal buttons to add more atoms. When you are finished, press Check Add metal Add nonmetal Show charge Lithium Fluorine Check Reset Controls: %3D Speed Tools
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter2: Lewis Structures
Section: Chapter Questions
Problem 14CTQ
Related questions
Question
Please paste a picture of lithium and fluorine on gizmos if you cant do that to i will delete the app because each tine i ask a question you guys say sorry we can not solve this question… why did i paid money for you guys if you cant solve anything!!?
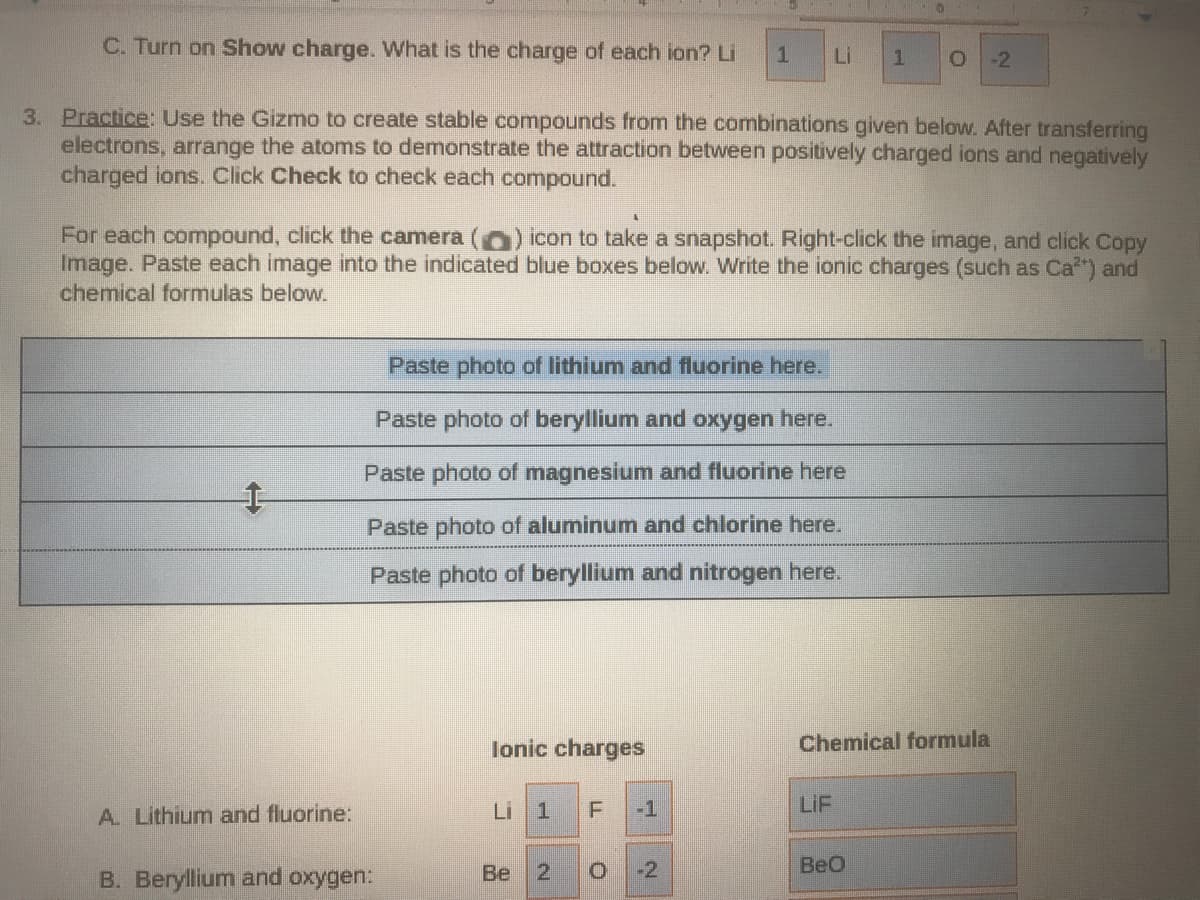
Transcribed Image Text:C. Turn on Show charge. VWhat is the charge of each ion? Li
Li
-2
3. Practice: Use the Gizmo to create stable compounds from the combinations given below. After transferring
electrons, arrange the atoms to demonstrate the attraction between positively charged ions and negatively
charged ions. Click Check to check each compound.
For each compound, click the camera ( ) icon to take a snapshot. Right-click the image, and click Copy
Image. Paste each image into the indicated blue boxes below. Write the ionic charges (such as Ca*) and
chemical formulas below.
Paste photo of lithium and fluorine here.
Paste photo of beryllium and oxygen here.
Paste photo of magnesium and fluorine here
Paste photo of aluminum and chlorine here.
Paste photo of beryllium and nitrogen here.
lonic charges
Chemical formula
A. Lithium and fluorine:
Li 1
-1
LIF
Be
21
-2
BeO
B. Beryllium and oxygen:
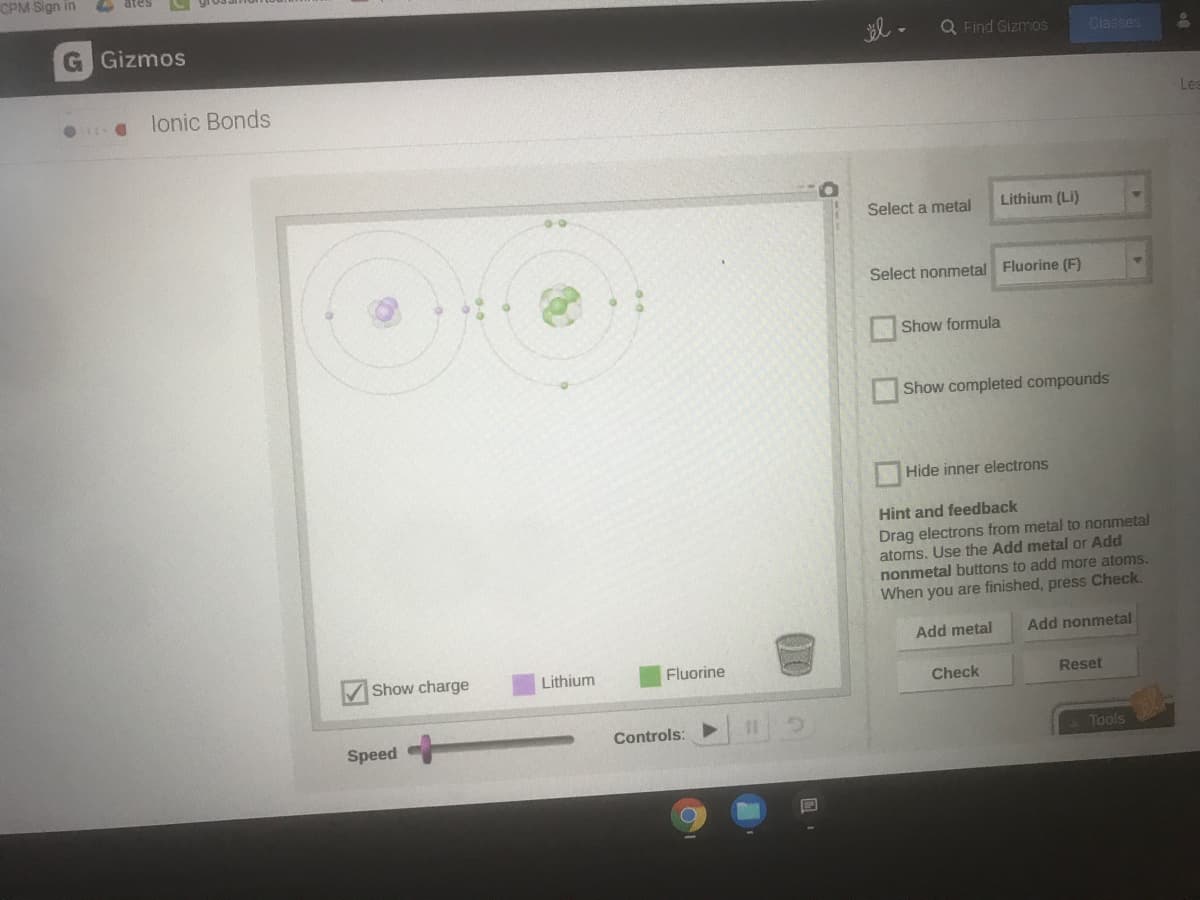
Transcribed Image Text:CPM Sign in
G Gizmos
Q Find Gizmos
Classes
lonic Bonds
Les
Select a metal
Lithium (Li)
Select nonmetal Fluorine (F)
Show formula
Show completed compounds
Hide inner electrons
Hint and feedback
Drag electrons from metal to nonmetal
atoms. Use the Add metal or Add
nonmetal buttons to add more atoms.
When you are finished, press Check.
Add metal
Add nonmetal
Show charge
Lithium
Fluorine
Check
Reset
Controls:
%3D
Speed
Tools
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning