g.com/ibiscms/mod/ibis/view.php?id:7679896 2) lofi hip hop radio-sad Bt s. Are el eaning counes for met riceline.com CH7Learning Objective.. At 850 K the equilibrium constant for the following reaction is Ke 15 If we mix the proceed toward of the three gases, predict in which direction the net reaction will No net reaction Left Right ISOl 0.80 M Ol-0.70 M ISOsl-0.16 M ISOJ 0.16M O2l-0.10 M SOsl-0.40 M ISO2l-020 M 021#060 M Previcus
g.com/ibiscms/mod/ibis/view.php?id:7679896 2) lofi hip hop radio-sad Bt s. Are el eaning counes for met riceline.com CH7Learning Objective.. At 850 K the equilibrium constant for the following reaction is Ke 15 If we mix the proceed toward of the three gases, predict in which direction the net reaction will No net reaction Left Right ISOl 0.80 M Ol-0.70 M ISOsl-0.16 M ISOJ 0.16M O2l-0.10 M SOsl-0.40 M ISO2l-020 M 021#060 M Previcus
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 76AP: Methanol can be synthesized by means of the equilibriumreaction CO(g)+2H2(g)CH3OH(g) for which the...
Related questions
Question
Please give me a step by step breakdown on how you get this answer.
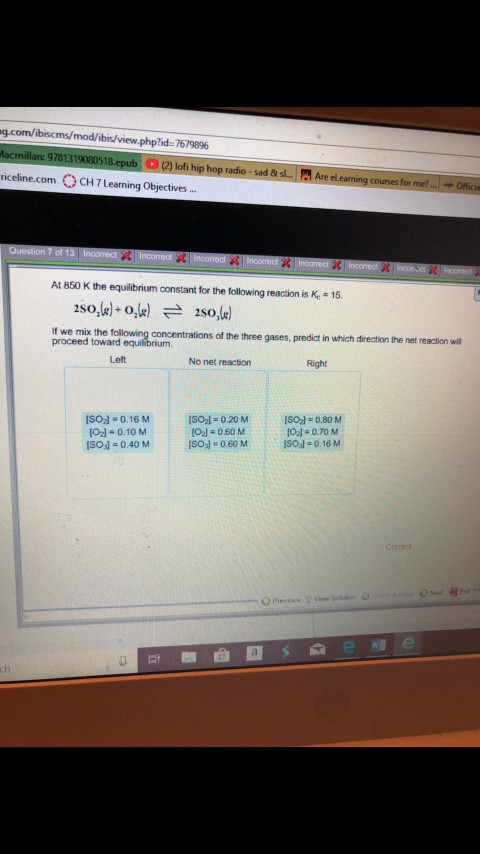
Transcribed Image Text:g.com/ibiscms/mod/ibis/view.php?id:7679896
2) lofi hip hop radio-sad Bt s.
Are el eaning counes for met
riceline.com CH7Learning Objective..
At 850 K the equilibrium constant for the following reaction is Ke 15
If we mix the
proceed toward
of the three gases, predict in which direction the net reaction will
No net reaction
Left
Right
ISOl 0.80 M
Ol-0.70 M
ISOsl-0.16 M
ISOJ 0.16M
O2l-0.10 M
SOsl-0.40 M
ISO2l-020 M
021#060 M
Previcus
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning