An increase in pressure favors a shift in the direction that will reduce the volume of the gas system, considering that the molecules are readily compressible. However, a change in solution pressure will have a negligible effect on the equilibrium. OA-If the first statement is TRUE and the second statement is FALSE OB-If the first statement is FALSE and the second statement is TRUE OC-If both statements are TRUE D-If both statements are FALSE
An increase in pressure favors a shift in the direction that will reduce the volume of the gas system, considering that the molecules are readily compressible. However, a change in solution pressure will have a negligible effect on the equilibrium. OA-If the first statement is TRUE and the second statement is FALSE OB-If the first statement is FALSE and the second statement is TRUE OC-If both statements are TRUE D-If both statements are FALSE
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.116PAE
Related questions
Question
Transcribed Image Text:46 46 11:02 b
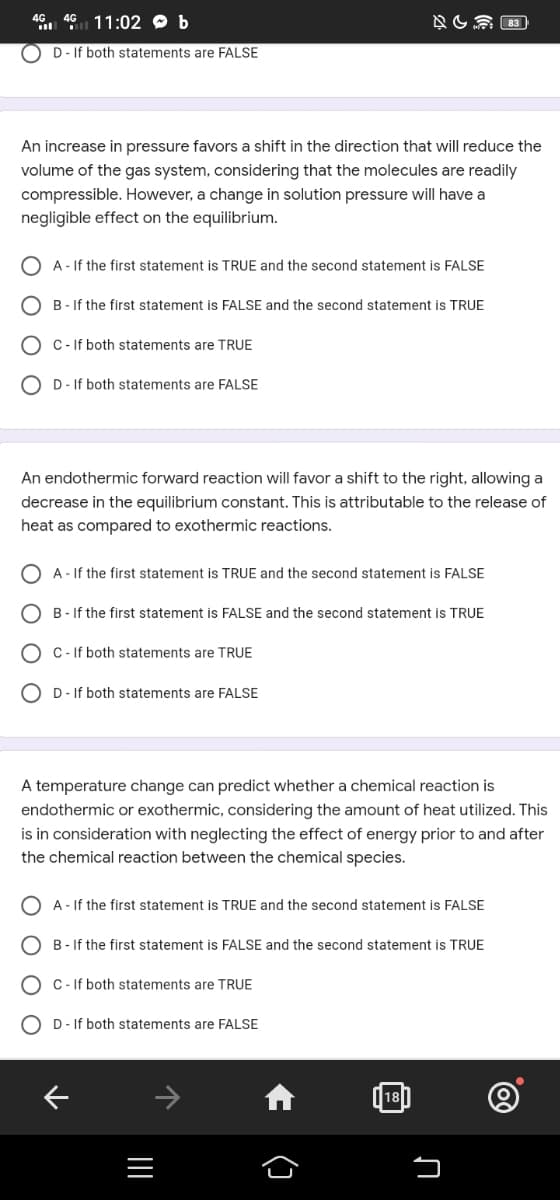
D - If both statements are FALSE
An increase in pressure favors a shift in the direction that will reduce the
volume of the gas system, considering that the molecules are readily
compressible. However, a change in solution pressure will have a
negligible effect on the equilibrium.
A - If the first statement is TRUE and the second statement is FALSE
B-If the first statement is FALSE and the second statement is TRUE
C - If both statements are TRUE
OD- If both statements are FALSE
An endothermic forward reaction will favor a shift to the right, allowing a
decrease in the equilibrium constant. This is attributable to the release of
heat as compared to exothermic reactions.
A - If the first statement is TRUE and the second statement is FALSE
B-If the first statement is FALSE and the second statement is TRUE
C-If both statements are TRUE
D - If both statements are FALSE
NU
A temperature change can predict whether a chemical reaction is
endothermic or exothermic, considering the amount of heat utilized. This
is in consideration with neglecting the effect of energy prior to and after
the chemical reaction between the chemical species.
A - If the first statement is TRUE and the second statement is FALSE
B-If the first statement is FALSE and the second statement is TRUE
C- If both statements are TRUE
DIf both statements are FALSE
↓
|||
()
|18|
♫
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning