gen cyanide, HCN, is a poisonous gas. The lethal dose is ximately 330 mg mg HCN per kilogram of air when d Part A Calculate the amount of HCN that gives the lethal dose in a small laboratory room measuring 12 x 14 x 8.0ft. The density of air at 20°C is 0.00118g/cm Express your answer using two significant figures. IVE ΑΣΦΑ m. Submit Previous Answers Request Answer X Incorrect, Try Again; 4 attempts remaining Part B the HCN is formed by reaction of NaCN with an acid such as H₂SO,, what mass of NaCN gives the lethal dose in the room 2NaCN(a)+ H₂SO,(og)-+Na, 80,(aq) + 2HCN() Express your answer using two significant figures. ma K VAX 4
gen cyanide, HCN, is a poisonous gas. The lethal dose is ximately 330 mg mg HCN per kilogram of air when d Part A Calculate the amount of HCN that gives the lethal dose in a small laboratory room measuring 12 x 14 x 8.0ft. The density of air at 20°C is 0.00118g/cm Express your answer using two significant figures. IVE ΑΣΦΑ m. Submit Previous Answers Request Answer X Incorrect, Try Again; 4 attempts remaining Part B the HCN is formed by reaction of NaCN with an acid such as H₂SO,, what mass of NaCN gives the lethal dose in the room 2NaCN(a)+ H₂SO,(og)-+Na, 80,(aq) + 2HCN() Express your answer using two significant figures. ma K VAX 4
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter1: Chemistry And Measurement
Section: Chapter Questions
Problem 1.164QP: Zinc ore (zinc sulfide) is treated with sulfuric acid, leaving a solution with some undissolved bits...
Related questions
Question
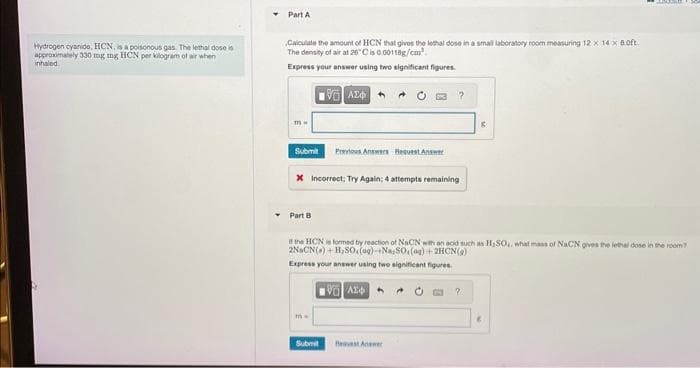
Transcribed Image Text:Hydrogen cyanide, HCN, is a poisonous gas. The lethal dose is
approximately 330 mg mg HCN per kilogram of air when
inhaled
Part A
Calculate the amount of HCN that gives the lethal dose in a small laboratory room measuring 12 x 14 x 8.0ft.
The density of air at 26°C is 0.00118g/cm²
Express your answer using two significant figures.
m=
Submit
LIVE ΑΣΦΑ
Part B
X Incorrect; Try Again: 4 attempts remaining
ma
Previous Answers Request Answer
the HCN is formed by reaction of NaCN with an acid such as H₂SO₂, what mass of NaCN gives the lethal dose in the room?
2NaCN()+ H₂SO.(ag)-Na,SO, (aq) + 2HCN(9)
Express your answer using two significant figures.
Submit
?
AL
Request Answer
2
E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning