Gibbs Free Energy for Nonspontaneous Reactions and Reactions at Equilibrium Consider the given Ellingham diagram. Rank the oxides of Mg, Zn, and Al in terms of increasing stability at 1000 ºC (1 being the least stable, 3 being the most stable).
Gibbs Free Energy for Nonspontaneous Reactions and Reactions at Equilibrium Consider the given Ellingham diagram. Rank the oxides of Mg, Zn, and Al in terms of increasing stability at 1000 ºC (1 being the least stable, 3 being the most stable).
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter9: Atomic Absorption And Atomic Fluorescence Spectrometry
Section: Chapter Questions
Problem 9.22QAP: The chromium in an aqueous sample was determined by pipetting 10.0 ml. of the unknown into each of...
Related questions
Question
Gibbs Free Energy for Nonspontaneous Reactions and Reactions at Equilibrium
Consider the given Ellingham diagram. Rank the oxides of Mg, Zn, and Al in terms of increasing stability at 1000 ºC (1 being the least stable, 3 being the most stable).
Transcribed Image Text:1
Al:O3
Zno
Mgo
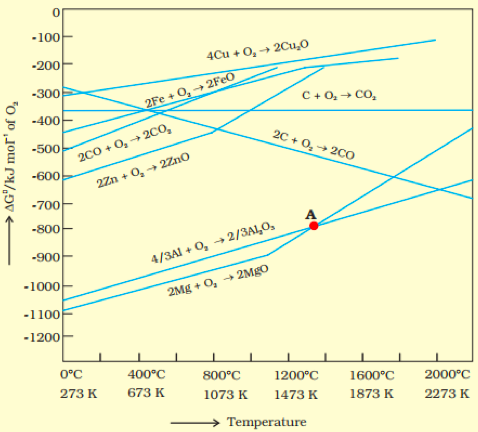
Transcribed Image Text:-100
-200
4Cu + O,→ 2Cu,0
-300
2Fe + 0, 2FEO
C+ 0,→ CO,
-400
-500
20 + 0,+ 20O
200 + 0, + 200,
-600
2Zn + 0, 2Zno
-700
A
-800
-900
4/3A1 + 0, → 2/3AĻO,
-1000
2Mg + O, + 2Mgo
-1100
-1200-
0°C
400°C
800°C
1200°C
1600°C
2000°C
273 K
673 K
1073 K
1473 K
1873 K
2273 K
Temperature
AG"/kJ mor' of O,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning