(a) Calculate the standard free-energy change and the equilibrium constant for the dimerization of NO, to N2O4 at 25°C (see Appendix D). (b) Calculate AG for this reaction at 25°C when the pres- sures of NO2 and N,O4 are each held at 0.010 atm. Which way will the reaction tend to proceed?
(a) Calculate the standard free-energy change and the equilibrium constant for the dimerization of NO, to N2O4 at 25°C (see Appendix D). (b) Calculate AG for this reaction at 25°C when the pres- sures of NO2 and N,O4 are each held at 0.010 atm. Which way will the reaction tend to proceed?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter18: Thermodynamics And Equilibrium
Section: Chapter Questions
Problem 18.99QP: a Calculate K1, at 25C for phosphoric acid: H3PO4(aq)H+(aq)+H2PO4(aq) b Which thermodynamic factor...
Related questions
Question
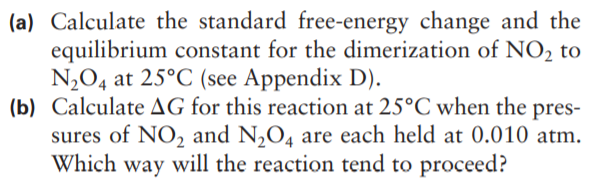
Transcribed Image Text:(a) Calculate the standard free-energy change and the
equilibrium constant for the dimerization of NO, to
N2O4 at 25°C (see Appendix D).
(b) Calculate AG for this reaction at 25°C when the pres-
sures of NO2 and N,O4 are each held at 0.010 atm.
Which way will the reaction tend to proceed?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning