
Concept explainers
Which of the following molecules is(are) polar? For each polar molecule indicate the direction of polarity—that is, which is the negative end, and which is the positive end of the molecule.
- (a) BeCl2
- (b) HBF2
- (c) CH3Cl
- (d) SO3
(a)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole moment in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
Explanation of Solution
(b)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole moment in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
Explanation of Solution
The structure of
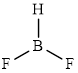
This molecule has a trigonal planar geometry. The fluorine atoms occupied on the negative end of the dipole and the hydrogen atom on the positive end.

Thus,
(c)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end (dipole) and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
Explanation of Solution
The structure of
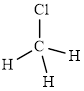
This molecule has a tetrahedral geometry. The chlorine atom occupied on the negative end of the dipole and the hydrogen atom on the positive end.
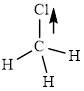
Thus,
(d)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole moment in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
Explanation of Solution
The structure of
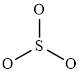
This molecule has trigonal planer geometry. The direction dipole moment in the molecule is represented below,
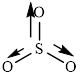
Thus,
However
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry & Chemical Reactivity
- Given the bonds C N, C H, C Br, and S O, (a) which atom in each is the more electronegative? (b) which of these bonds is the most polar?arrow_forwardWhich of the following molecules contain a triple bond in the image below.arrow_forwardWhich model, the ball-and-stick or the space-filling, more effectively showsthe angles between bonds around a central atom?arrow_forward
- What is the shape and polarity for the molecule A3 - where A is a halogen?arrow_forwardIn the actual structure of acetic acid, which bond angle isexpected to be the smallest?arrow_forwardHow many different structural isomers are there for octahedral molecules with the general formula AX2Y3 if A is the inner atom and there are no X-Y, X-X and Y-Y bonds?arrow_forward
- Complete the following electron-dot structure for Methylphenidate (C14H19NO2), showing the position of any multiple bonds and lone pairs.arrow_forwardThe partial Lewis structure that follows is for a hydrocarbonmolecule. In the full Lewis structure, each carbon atomsatisfies the octet rule, and there are no unshared electronpairs in the molecule. The carbon—carbon bondsare labeled 1, 2, and 3. (a) How many hydrogen atomsare in the molecule? (b) Rank the carbon–carbonbonds in order of increasing bond length. (c) Whichcarbon—carbon bond is the strongest one?arrow_forwardFor SO3 provide a Lewis structure, predicted VSEPR molecular geometry, bond angle and indicat whether the compound is polar, no polar or a polyatomic ion.arrow_forward
- (a) Methane (CH4) and the perchlorate ion (ClO4- ) are bothdescribed as tetrahedral. What does this indicate about theirbond angles? (b) The NH3 molecule is trigonal pyramidal, while BF3 is trigonal planar. Which of these molecules is flat?arrow_forwardWhich of the following statements could be true regarding polar molecules?arrow_forwardConsider the collection of nonmetallic elements O, P, Te,I, and B. (a) Which two would form the most polar singlebond? (b) Which two would form the longest single bond?(c) Which two would be likely to form a compound of formulaXY2? (d) Which combinations of elements would likelyyield a compound of empirical formula X2Y3?arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning



