Given the absorbance spectrum shown below, what wavelength of light would you use for nickel(II) ion? Explain your answer. Absorbance vs. Wavelength for Nickel(11) ion 0.8 0.6 0.4 0.2 0. 350 400 450 500 550 600 650 700 750 800 85 Wavelength (nm)
Given the absorbance spectrum shown below, what wavelength of light would you use for nickel(II) ion? Explain your answer. Absorbance vs. Wavelength for Nickel(11) ion 0.8 0.6 0.4 0.2 0. 350 400 450 500 550 600 650 700 750 800 85 Wavelength (nm)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter9: Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals
Section9.3: Theories Of Chemical Bonding: A Summary
Problem 2.2ACP: Butter yellow absorbs light with a wavelength of 408 nm, whereas the nitrated form absorbs at 478...
Related questions
Question
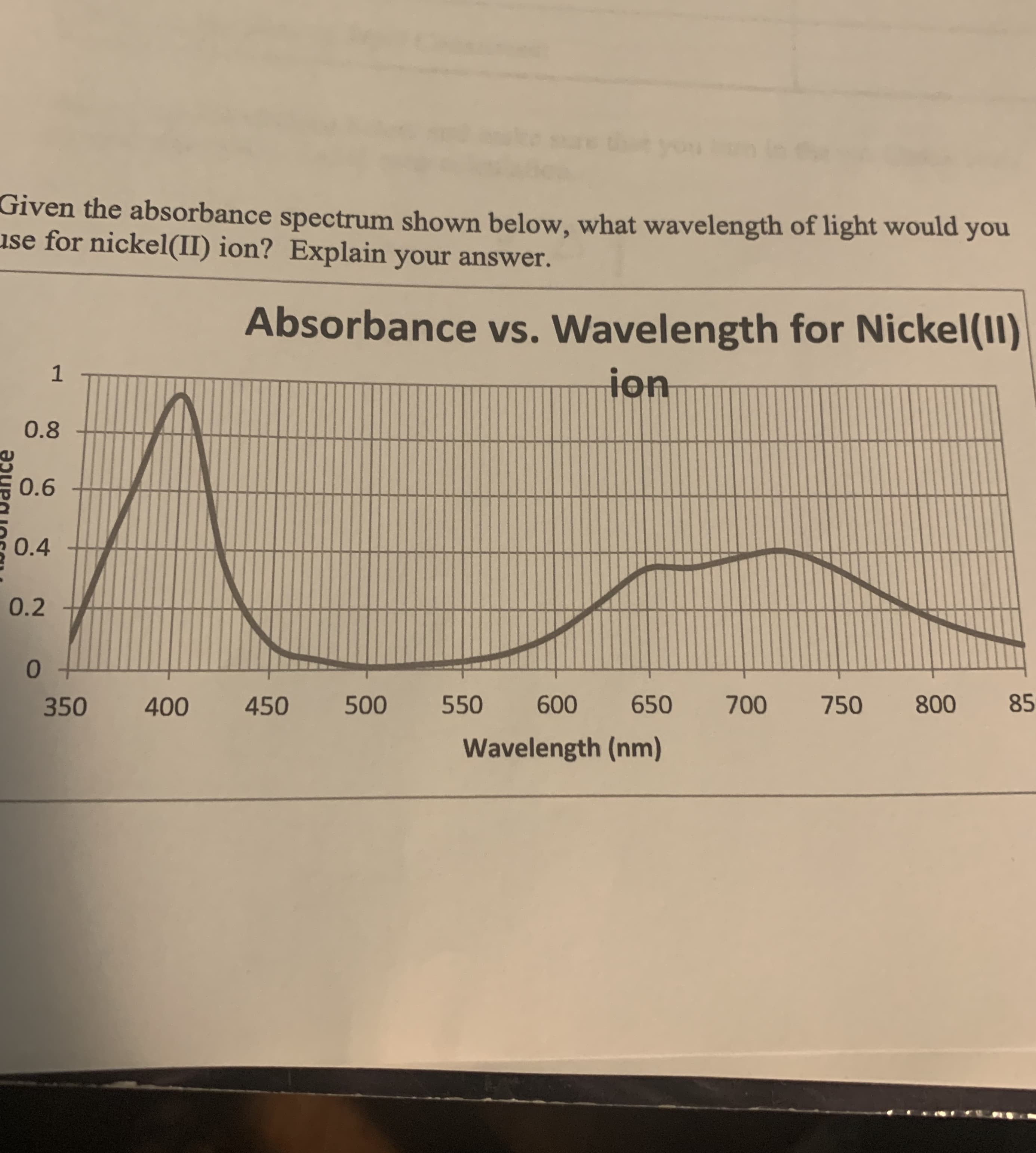
Transcribed Image Text:Given the absorbance spectrum shown below, what wavelength of light would you
use for nickel(II) ion? Explain your answer.
Absorbance vs. Wavelength for Nickel(11)
ion
0.8
0.6
0.4
0.2
0.
350
400
450
500
550
600
650
700
750
800
85
Wavelength (nm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning