Given the molecular formula and ¹³C NMR data (in ppm), deduce and draw the structure of the unknown compound. The type of carbon, as revealed from DEPT spectra, is specified in each case. Molecular Formula C₂H6 Draw the unknown compound. DEPT data 30.2 (CH₂), 136.0 (CH)
Given the molecular formula and ¹³C NMR data (in ppm), deduce and draw the structure of the unknown compound. The type of carbon, as revealed from DEPT spectra, is specified in each case. Molecular Formula C₂H6 Draw the unknown compound. DEPT data 30.2 (CH₂), 136.0 (CH)
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 14AP: Propose structures for compounds that fit the following mass-spectral data: (a) A hydrocarbon with...
Related questions
Question
Feedback: While the structure you've drawn fits the molecular formula, would give two carbon resonances, and fits the DEPT data, it does not fit the chemical shift of the CH2 carbon.
The low chemical shift suggests an aliphatic CH2.
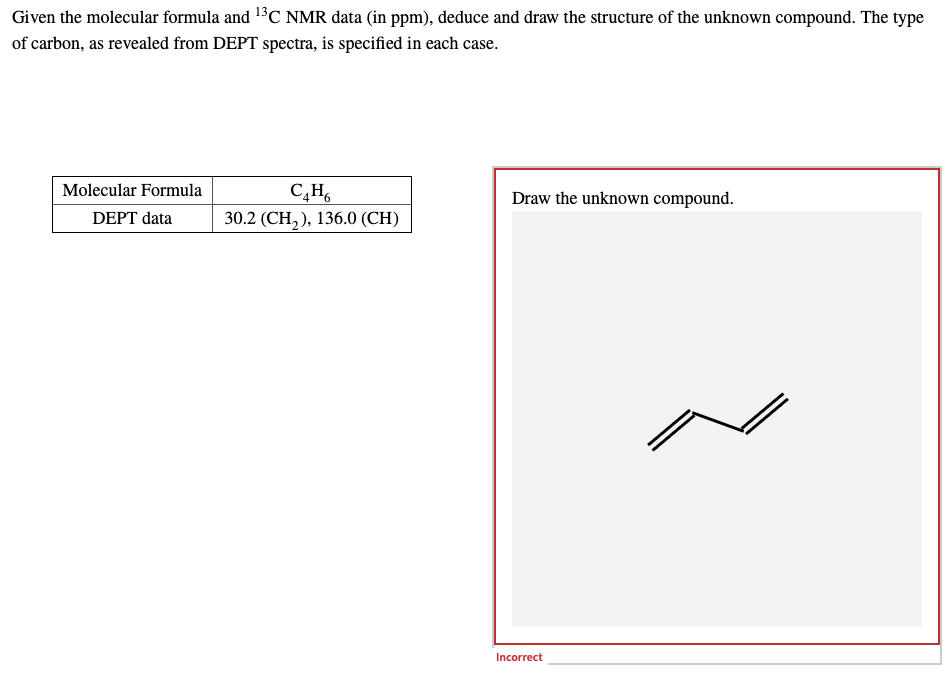
Transcribed Image Text:Given the molecular formula and ¹³C NMR data (in ppm), deduce and draw the structure of the unknown compound. The type
of carbon, as revealed from DEPT spectra, is specified in each case.
Molecular Formula
Draw the unknown compound.
C₂H6
30.2 (CH₂), 136.0 (CH)
DEPT data
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning