Given the spectra below, answer the following questions, both spectra were obtained with 1.0 cm optical path: (I) Which region is being studied (II)G and 6 T-G are molecules or atoms, justify your answer (III) What is λmax of G and 6 GT? (IV) If G has a concentration of 0.25 mM, what is its absorptivity at 250 nm? (V) If G has a concentration of 0.25 mM, what is its absorptivity at 350 nm (VI) If 6 GT has a concentration of 0.4 mM, what is your absorptivity at 250 nm? (VII) If G has a concentration of 0.24 mM, what is your absorptivity at 350 nm? (VIII) One solution contains 0.42 mM G and 0.03 mM 6 GT, what will be the absorbance of this solution in 250 and 350 nm ?
Given the spectra below, answer the following questions, both spectra were obtained with 1.0 cm optical path: (I) Which region is being studied (II)G and 6 T-G are molecules or atoms, justify your answer (III) What is λmax of G and 6 GT? (IV) If G has a concentration of 0.25 mM, what is its absorptivity at 250 nm? (V) If G has a concentration of 0.25 mM, what is its absorptivity at 350 nm (VI) If 6 GT has a concentration of 0.4 mM, what is your absorptivity at 250 nm? (VII) If G has a concentration of 0.24 mM, what is your absorptivity at 350 nm? (VIII) One solution contains 0.42 mM G and 0.03 mM 6 GT, what will be the absorbance of this solution in 250 and 350 nm ?
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.16QAP
Related questions
Question
Given the spectra below, answer the following questions, both spectra were obtained with 1.0 cm optical path:
(I) Which region is being studied
(II)G and 6 T-G are molecules or atoms, justify your answer
(III) What is λmax of G and 6 GT?
(IV) If G has a concentration of 0.25 mM, what is its absorptivity at 250 nm?
(V) If G has a concentration of 0.25 mM, what is its absorptivity at 350 nm
(VI) If 6 GT has a concentration of 0.4 mM, what is your absorptivity at 250 nm?
(VII) If G has a concentration of 0.24 mM, what is your absorptivity at 350 nm?
(VIII) One solution contains 0.42 mM G and 0.03 mM 6 GT, what will be the absorbance of this solution in 250 and 350 nm ?
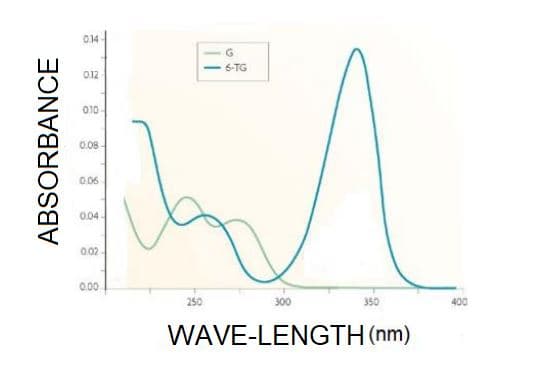
Transcribed Image Text:014
6-TG
012
010
0.08-
0.06
04
0.02
0.00
250
300
350
400
WAVE-LENGTH (nm)
ABSORBANCE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning