1. b. Given that a methyl group (-CH3) is bulkier than a (-F) group, will the J13C-1H coupling constant for the H atoms bound to the central carbon of compound 2 (indicated with the red circle) be greater than or less than 184.5 Hz? Provide your reasoning. H H) "CH3 H3C H F 1 1. c. Which of the compounds given in question 1B has a higher pKa? For the more acidic compound, write the chemical reaction that defines its pKa and give the equation defining the relevant equilibrium constant.
1. b. Given that a methyl group (-CH3) is bulkier than a (-F) group, will the J13C-1H coupling constant for the H atoms bound to the central carbon of compound 2 (indicated with the red circle) be greater than or less than 184.5 Hz? Provide your reasoning. H H) "CH3 H3C H F 1 1. c. Which of the compounds given in question 1B has a higher pKa? For the more acidic compound, write the chemical reaction that defines its pKa and give the equation defining the relevant equilibrium constant.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL1: Infrared Spectroscopy
Section: Chapter Questions
Problem 13CTQ
Related questions
Question
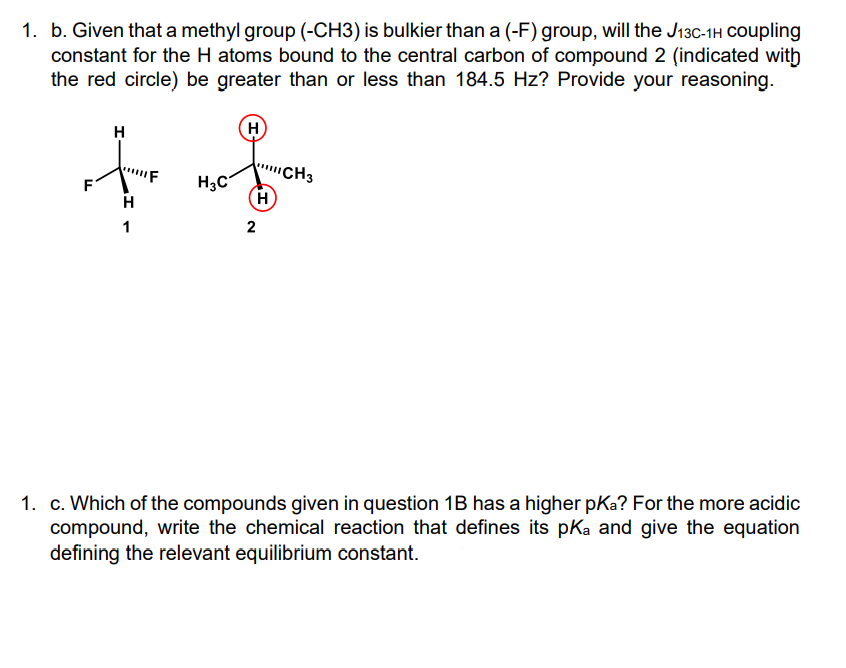
Transcribed Image Text:1. b. Given that a methyl group (-CH3) is bulkier than a (-F) group, will the J13C-1H Coupling
constant for the H atoms bound to the central carbon of compound 2 (indicated with
the red circle) be greater than or less than 184.5 Hz? Provide your reasoning.
H
CH3
H3C
H
F
H
1
2
1. c. Which of the compounds given in question 1B has a higher pKa? For the more acidic
compound, write the chemical reaction that defines its pKa and give the equation
defining the relevant equilibrium constant.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole