Given the two reactions 1. H2 S(aq) = HS-(aq)+ H+(aq), K1 = 9.38×10-8, and 2. HS (aq) = S²- (aq) +H†(aq), K2 = 1.16x10-19, what is the equilibrium constant Kfinal for the following reaction? S²-(aq) + 2H†(aq) = H2S(aq)
Q: | Find the equilbrium expression (Ka) for the ionization reaction. HCO3 (aq) + H20(1) SH30*(aq) +…
A:
Q: Calculate the Keq for this reaction if the KP = 78.3 at 100°C. 4 HCl(g) + O2(g) ⇄ 2 H2O(g) +…
A:
Q: The acid-dissociation constant for chlorous acid 1HClO22 is 1.1 * 10-2. Calculate the concentrations…
A: Given: Initial Concentration of HClO2 = 0.0125 M
Q: Vinegar sold commercially is typically 0.8−1.0 M acetic acid. A 1.00 M solution of acetic acid is…
A: The solution is as follows:
Q: Calculate the equilibrium constant for the following reaction: Ag2S(s) + 4 Cl- (aq) + 2 H+(aq) = 2…
A: The chemical equation for the solubility product of Ag2S involves the dissociation of Ag2S into its…
Q: Which of the following is not a protonated substance: a) HCl b) HA c) KOH d) HF
A:
Q: Given the equilibrium constants for the following two reactionsin aqueous solution at 25…
A: A reversible chemical reaction can move in either forward or backward direction. The stage of a…
Q: NH3(aq) + H2O (l) ⇆ NH4+(aq) + OH- (aq) 2. If the concentrations for reactants and products are:…
A: Solution -
Q: References] Obtain the equilibrium constant for the reaction HCN(aq) = H+ (aq) + CN¯(aq) From the…
A:
Q: Given the following equilibrium constants at 417°C, Na20(s) = 2 Na(1) + 02 (9) K1 = 3 x 10-2 1…
A:
Q: 1) O2 + 4NO2 2N2O5 K= 3.5 x 10° What concentration of N2O5 would be in equilibrium with 0.55M O2 and…
A: Nitrogen dioxide reacts with oxygen to form dinitrogen pentaoxide. The equilibrium reaction is as…
Q: Write the expression for the K₂ for each of the following reactions H₂CO3 + H₂O H3O+¹ + HCO3-¹ [ ][…
A:
Q: Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aqueous solution:…
A: The equation of dissociation is given as: HC2H3O2 (aq) --><-- C2H3O2- (aq) + H+ (aq) The…
Q: PbCl2(aq) Pb2+(aq) +2Cl− (aq), K = 1.83×10−10 AgCl(aq) Ag+(aq) + Cl− (aq) K = 1.29×10−4…
A:
Q: Struggling please help. Thanks in advance.
A: Hey, since there are multiple questions posted, we will answer first question. If you want any…
Q: Acetic acid (pK, = 4.76) was dissolved in an aqueous solution buffered to a pH of 5.76. Determine…
A: The ratio of concentration of acetate ion and acetic acid can be calculated using Henderson…
Q: Given the following two equilibra: NiCO3(s)----> Ni2(aq)+ CO32-(aq). K1=6.6x10-9 HCO3-(aq)+H2O(l)…
A: Given: K1 = 6.6×10-9 K2= 4.8×10-11
Q: The acid-dissociation constant for benzoic acid (C6H5COOH)is 6.3 x 10-5. Calculate the equilibrium…
A: The dissociation reaction of C6H5COOH is as shown below => C6H5COOH (aq) + H2O (l) ------>…
Q: Ammonia reacts with water to produce ammonium ion and hydroxide ion as shown in the equation below.…
A:
Q: One way to determine the predominant species at equilibrium for an acid-base reaction is to say that…
A: The pKa value of species in an equilibrium is a useful factor to determine the predominant species…
Q: 8. (11.7) Using the following reactions at 298 K: CH,CHO + 2H + 2e CH,CH;OH E - -0.197 NAD + H + 2e…
A: Hello. Since your question has multiple parts, we will solve the first question for you. If you want…
Q: 1) Production of a diprotic acid H2XO4 involves 3 steps as shown below: 1. X(s) + O2(g) = XO2 (g)…
A: Since, you have asked multiple question, we will solve the first question for you. If you want any…
Q: R-NH2 (aq) + H20(1) = OH" (aq) + R-NH; (aq) AH<0
A: Equilibrium constant is the ratio of product of active mass of products at numerator with product of…
Q: The following equilibrium constants have been determined for hydrosulfuric acid at 25oC ;…
A:
Q: Obtain the equilibrium constant for the reaction HCN(ag) - H* (ag) + CN (ag) From the following:…
A: When two chemical equations are added to get the net chemical equation, then the equilibrium…
Q: Oxalic acid ionizes in two stages in aqueous solution: H2C2O4(aq) + H2O(€ )2H;0* (aq) + HC2O4 (aq)…
A: Consider the ionization of sodium oxalate in water as shown below. C2O42-+H2O↔HC2O4-+OH- The ICE…
Q: . [A] is 0.1 M solution is 6.5 % ionized. Calculate the equilibrium constant of the compound. A(g) +…
A:
Q: Calculate the value of ΔG∘rxnΔGrxn∘ for the following reaction at 294 K. Ka = 2.9 × 10–8 and assume…
A: The reaction given is, => HClO (aq) + H2O (l) -----------> ClO- (aq) + H3O+ (aq)…
Q: Given the two reactions 3. PBC12 (aq) = Pb²+(aq) + 2Cl-(aq), K3 = 1.86×10-10, and 4. AgCl(aq) =…
A: Given: Two reactions, PbCl2(aq)⇌Pb2+(aq)+2Cl-(aq) ; K3=1.86×10-10 AgCl(aq)⇌Ag+(aq)+Cl-(aq) ;…
Q: List acetic acid, chlorous acid, hydrofluoric acid and nitrous acid in order of increasing strength…
A:
Q: Caz (aq,). + C 2042- Caq,) CaC 204 (5) Kc =2.3x 101 1. Explain the components af the eguilibnium…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: 2. The popular antacid, Milk of Magnesia, is a suspension of magnesium hydroxide, Mg(OH)2. In water,…
A:
Q: 1.) Which of the following is the correct equilibrium constant for the following KC1O4 (s) + HCI…
A:
Q: Calculate the value of Δ?∘rxnΔGrxn∘ for the following reaction at 278 K. Ka = 2.9 × 10–8 and assume…
A: delta G is known as free energy of thermodynamical system , it is used to find maximum reversible…
Q: 9. Which equilibrium constant would be most relevant to the following system in equilibrium:…
A: Given: HC2H3O2 (aq) )⇌H(aq) ++C2H3O2-(aq) To find: relevant equilibrium constant.
Q: The following equilibrium constants have been determined for hydrosulfuric acid at 25°C: H2S(aq)…
A:
Q: The value of Ka for nitrous acid (HNO2) at 25 ∘C is 4.5×10-4 △G°=19.1kJ What is the value of △G…
A: The spontaneous nature of the reaction is determined by calculating the change of free energy of the…
Q: The equalibrium constant for the following equation HC2H3O2(aq) + H2O(l) ⇌…
A: The reaction taking place is given as, => HC2H3O2 (aq) + H2O ⇌ H3O+ (aq) + C2H3O2- (aq)
Q: Kc for formic acid dissociation in water is 1.8x10-4. What are the equilibrium concentrations of…
A:
Q: Obtain the equilibrium constant for the reaction HCN (aq) ---- H+(aq) +CN- (aq) From the following:…
A:
Q: The equilibrium constant for the reaction C6H5COOH(aq) + CH3COO"(aq) s C6H5CO0¯(aq) + CH3COOH(aq) is…
A:
Q: Calculate the equilibrium constant for the following reaction: H2SO3 (aq) =2 H+ (aq) + (SO3)2- (aq)…
A:
Q: Calculate the equilibrium constant at 25.0 oC for the following equation. Cr2O7-2(aq) + 6Fe+2(aq)…
A: The half reactions are -
Q: Prepare this aqueous mixture: 300 mL 0.1 M HNO2 & 100 mL 0.1 M PO4 3- a. HNO2 is in excess & PO43-…
A: For, H3PO4 Ka1 = 7.1×10-3 Ka2 =6.3×10-8 Ka3 = 7.1×10-13 Ka of HNO2 = 5×10-4 (a) PO43- + HNO2 ⇌HPO42-…
Q: Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aqueous solution:…
A: The equilibrium constant of a reaction is calculated by the help of equilibrium concentrations of…
Q: What is the relationship between the equilibrium constants for reactions 1, 2, and 3? K₁ Reaction 1:…
A: Dear student , since you have posted multiple questions we will allow to solve only first question…
Q: 2. When acetic acid (HC2H3O2) dissolves in water, the following reaction happens: HC;H;O2(aq) H,O…
A: The equilibrium reaction given is, Given: Concentration of acetic acid taken = 0.100 M.
Q: Reacting GeO2 with H20 forms the tetraprotic (four H* ions) acid, H4GEO4. On a piece of paper, write…
A: Conjugate acid is made when we add H+ ion on the given compound And conjugate base is made when we…
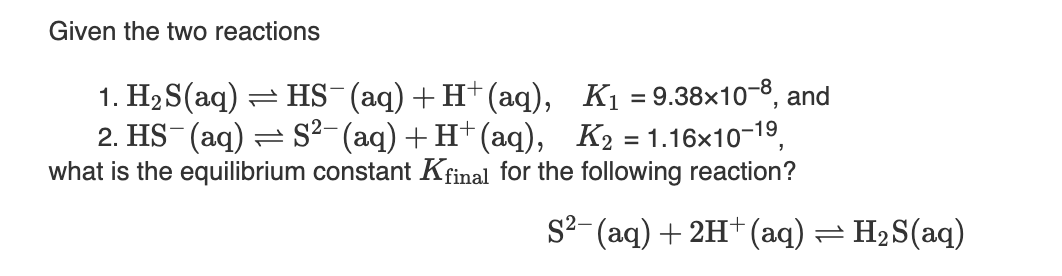
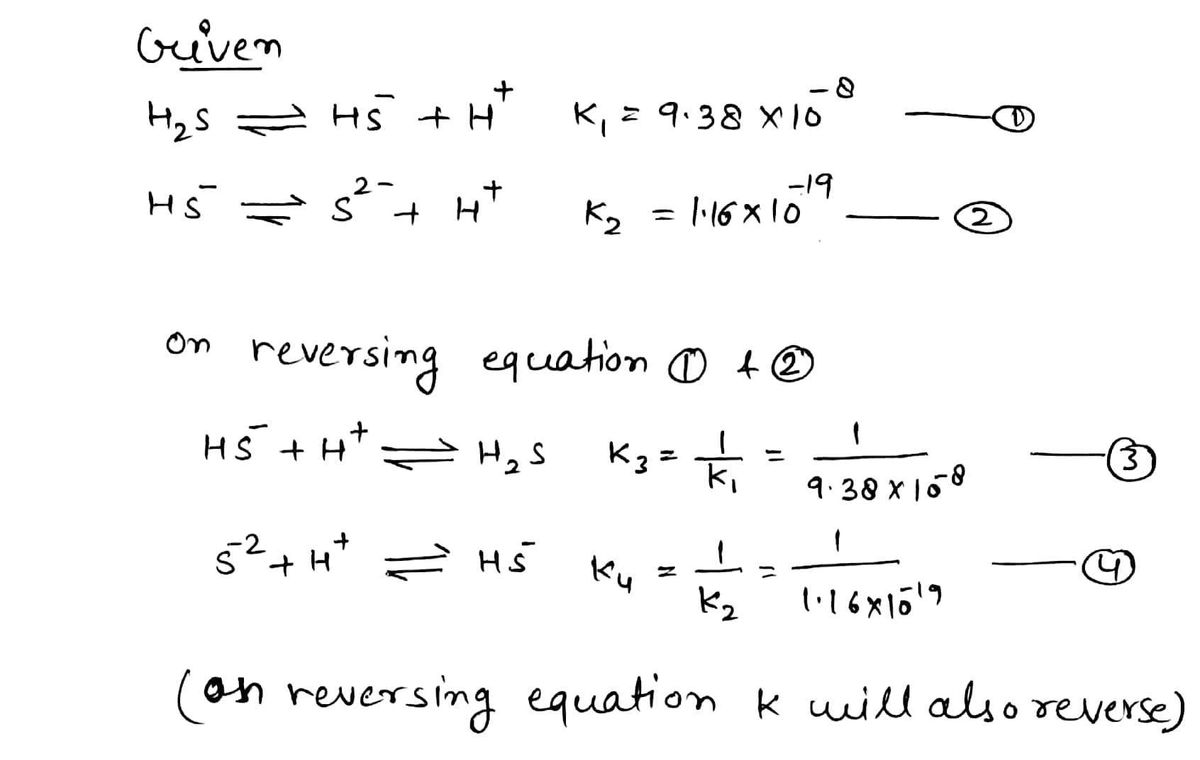
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- You have found the following: CaSO4(s) <=> Ca+2(aq) + SO4-2(aq) K = (6.053x10^-5) What is the value of K for the following reaction? 2 CaSO4(s) <=> 2 Ca+2(aq) + 2 SO4-2(aq) Note: Your answer is assumed to be reduced to the highest power possible. Please provide neat and clean handwriting and clear image Give answer correctlyThe acid-dissociation constant for chlorous acid (HClO2) is1.1 x 10-2. Calculate the concentrations of H3O+, ClO2-,and HClO2 at equilibrium if the initial concentration ofHClO2 is 0.0125 M.Given the following two equilibra: NiCO3(s)----> Ni2(aq)+ CO32-(aq). K1=6.6x10-9 HCO3-(aq)+H2O(l) CO32-(aq) +H3O+(aq). K2= 4.8x10-11 Calculate the equilibrium constant for the following reaction: NiCO3(s)+H3O+(aq)---->Ni2+(aq)+HCO3-(aq)+H2O(l) K3=?
- Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aqueous solution: HC2H3O2 (aq) --><-- C2H3O2- (aq) + H+ (aq) At equilibrium at 25 °C a 0.100 M solution of acetic acid has the following concentrations: [HC2H3O2] = 0.0990 M, [C2H3O2-] = 1.33 × 10-3 M and [H+] = 1.33 × 10-3 M. The equilibrium constant, Keq, for the ionization of acetic acid at 25 °C is ________.What is ∆G0 for the reaction CN- + H2O = HCN + OH-? Ka for HCN = 4.0x10-10. R = 8.314 J/(mol*K)The equilibrium at 25 °C MaCl5 (aq) + 4 H2O (l) ↔ H3MaO4 (aq) + 5 HCl (aq) Kc = 5500 ΔH°(rxn) = 28.1 kJ/mol, Determine the ΔG°(rxn)
- Given the two reactions H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.11×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.46×10−19, what is the equilibrium constant Kfinal for the following reaction? S2−(aq)+2H+(aq)⇌H2S(aq) Enter your answer numerically.Consider the following aqueous chemical equilibrium of benzoic acid, a weak acid: C6H5COOH(aq) + H2O(l) is in equilibrium with H3O+(aq) + C6H5COO-(aq) a. The addition of H3O+(aq) to the chemical equilibrium will have what effect on the amount (moles) of C6H5COO-(aq) in the system? b. Addition of OH-(aq) to the chemical equilibrium will have what effect on the amount (moles) of C6H5COOH(aq) in the system? c. Removal of C6H5COO-(aq) from the chemical equilibrium will have what effect on the amount of (moles) H3O+(aq) in the system? d. Increasing the pH of the solution will have what effect on the amount (moles) of C6H5COOH(aq) in the system?A) Write the equalibrium expression for N2 + 3H2----> 2NH3 NH3= .2 N2= .01 H2= .1 b) which is more favored the reactants or products
- Calculate the value of ΔG∘rxnΔGrxn∘ for the following reaction at 282 K. Ka = 2.9 × 10–8 and assume Ka does not change significantly with temperature. $$HClO(aq)+H2O(l)ClO−(aq)+H3O+(aq) kJ/molwrite the equilibrium constant experssion for thr following reactionsAcetic acid is a weak acid that dissociates into the acetate ion and a proton in aqueous solution: HC2H3O2(aq) + H2O(l) ↔ C2H3O2-(aq) + H3O+(aq). At equilibrium at 25 °C a 0.100 M solution of acetic acid has the following concentrations: [HC2H3O2]=0.0990 and [C2H3O2-]=1.33×10-3M, and [H3O+]=1.33×10-3M,. The equilibrium constant, Keq, for the ionization of acetic acid at 25 °C is __________.

