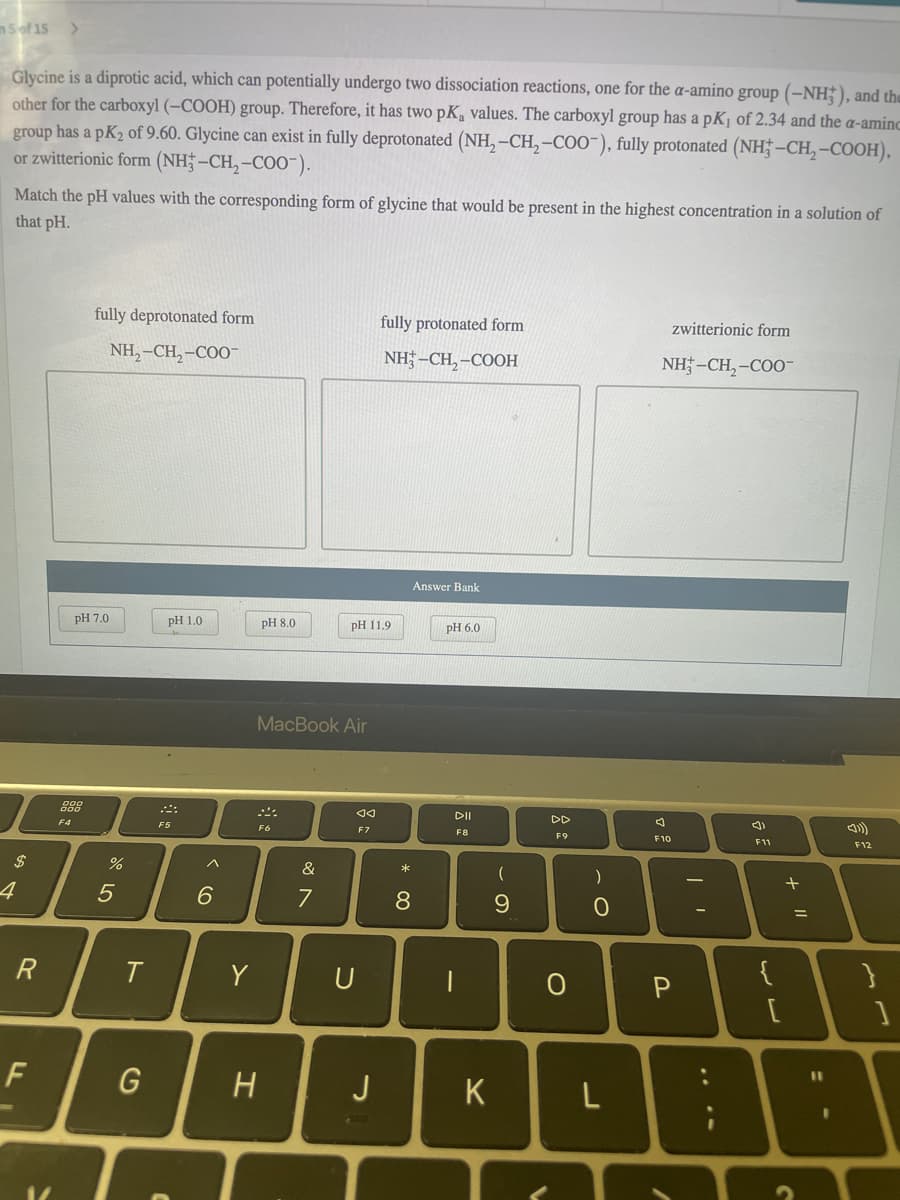
Glycine is a diprotic acid, which can potentially undergo two dissociation reactions, one for the a-amino group (-NH;), and the other for the carboxyl (-COOH) group. Therefore, it has two pK, values. The carboxyl group has a pKj of 2.34 and the a-amine group has a pK2 of 9.60. Glycine can exist in fully deprotonated (NH, -CH, -COO"), fully protonated (NH† -CH,-COOH), or zwitterionic form (NH;-CH,-COO-). Match the pH values with the corresponding form of glycine that would be present in the highest concentration in a solution of that pH.
Glycine is a diprotic acid, which can potentially undergo two dissociation reactions, one for the a-amino group (-NH;), and the other for the carboxyl (-COOH) group. Therefore, it has two pK, values. The carboxyl group has a pKj of 2.34 and the a-amine group has a pK2 of 9.60. Glycine can exist in fully deprotonated (NH, -CH, -COO"), fully protonated (NH† -CH,-COOH), or zwitterionic form (NH;-CH,-COO-). Match the pH values with the corresponding form of glycine that would be present in the highest concentration in a solution of that pH.
Chapter22: Carbonyl Alpha-substitution Reactions
Section22.SE: Something Extra
Problem 63AP: As far back as the 16th century, South American Incas chewed the leaves of the coca bush,...
Related questions
Question
Transcribed Image Text:n5of 15 >
Glycine is a diprotic acid, which can potentially undergo two dissociation reactions, one for the a-amino group (–NH;), and the
other for the carboxyl (–COOH) group. Therefore, it has two pK, values. The carboxyl group has a pKj of 2.34 and the a-aminc
group has a pK2 of 9.60. Glycine can exist in fully deprotonated (NH, –CH, –COO"), fully protonated (NH† –CH, -COOH),
or zwitterionic form (NH;-CH,-CO0-).
Match the pH values with the corresponding form of glycine that would be present in the highest concentration in a solution of
that pH.
fully deprotonated form
fully protonated form
zwitterionic form
NH,–CH,-COO"
NH; –CH,-COOH
NH -CH, -COO-
Answer Bank
pH 7.0
pH 1.0
pH 8.0
pH 11.9
PН 6.0
MacBook Air
DII
DD
F4
F5
F6
F7
F8
F9
F10
F11
F12
$
&
*
4
5
6.
7
8.
R
T.
Y
U
%D
F
H
J
K
+ ||
* 00
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning