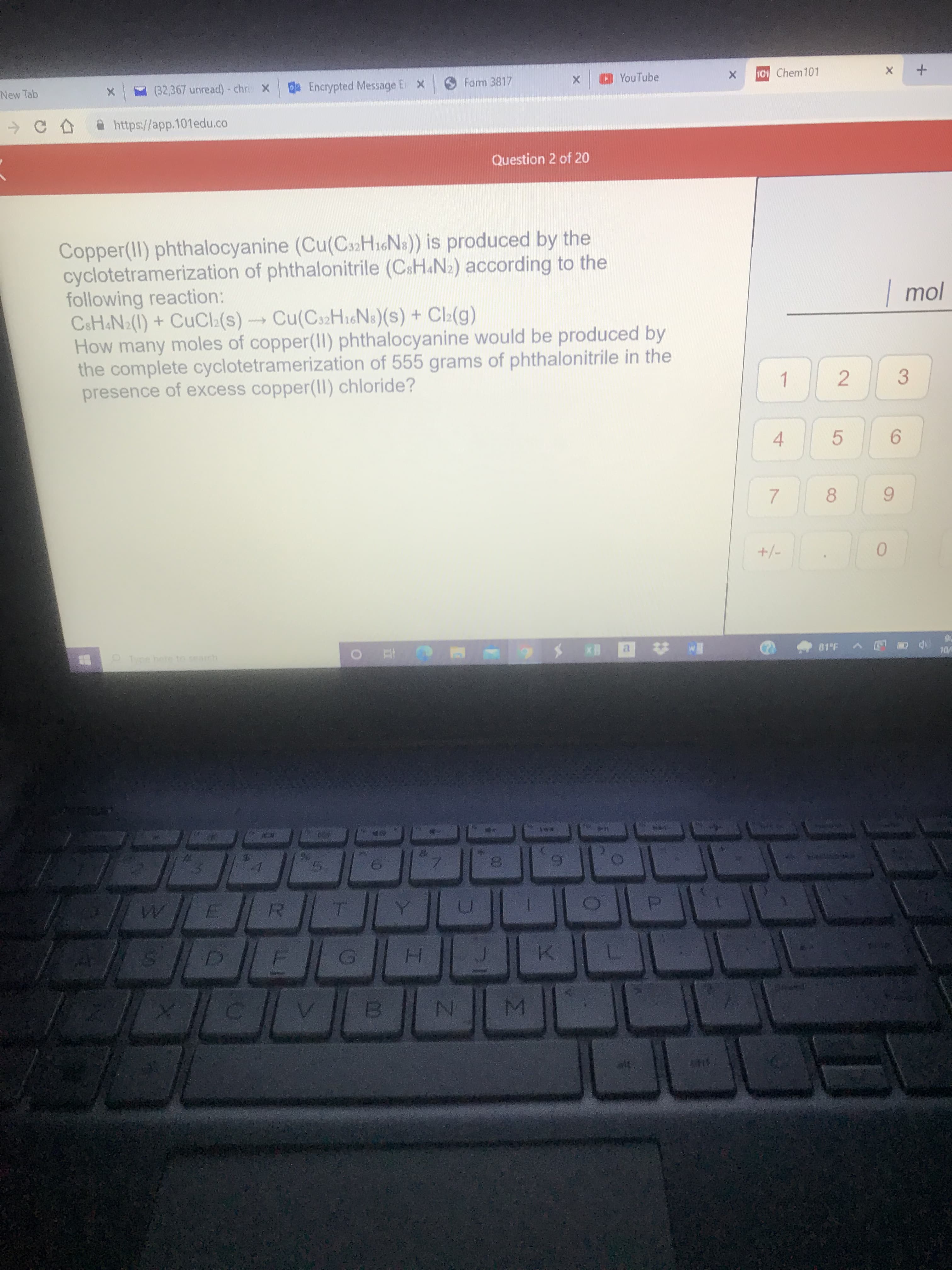
-> A https://app.101edu.co Question 2 of 20 Copper(II) phthalocyanine (Cu(Cs2HiGNs)) is produced by the cyclotetramerization of phthalonitrile (CsH.N2) according to the following reaction: CaH&N2(1) + CuCI:(s)Cu(C32H16N8)(s) + Cl(g) How many moles of copper(II) phthalocyanine would be produced by the complete cyclotetramerization of 555 grams of phthalonitrile in the presence of excess copper(II) chloride? 1 4. 2.
-> A https://app.101edu.co Question 2 of 20 Copper(II) phthalocyanine (Cu(Cs2HiGNs)) is produced by the cyclotetramerization of phthalonitrile (CsH.N2) according to the following reaction: CaH&N2(1) + CuCI:(s)Cu(C32H16N8)(s) + Cl(g) How many moles of copper(II) phthalocyanine would be produced by the complete cyclotetramerization of 555 grams of phthalonitrile in the presence of excess copper(II) chloride? 1 4. 2.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter22: Reactions Of Benzene And Its Derivatives
Section: Chapter Questions
Problem 22.51P
Related questions
Question
Transcribed Image Text:2.
7,
New Tab
(32,367 unread)- chr x
de Encrypted Message E X
6 Form 3817
YouTube
101 Chem101
A https://app.101edu.co
Question 2 of 20
Copper(II) phthalocyanine (Cu(Cs2HiGNs)) is produced by the
cyclotetramerization of phthalonitrile (CsH&N2) according to the
following reaction:
C&H&N2(1) + CUCI:(s)Cu(C32H16N:)(s) + Cl:(g)
How many moles of copper(II) phthalocyanine would be produced by
the complete cyclotetramerization of 555 grams of phthalonitrile in the
presence of excess copper(I) chloride?
| mol
3.
45
8.
K.
34
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning