Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter19: Nuclear Magnetic Resonance Spectroscopy
Section: Chapter Questions
Problem 19.12QAP
Related questions
Question
predict ir speck for the starting product
i made an example og how it needs to look like and linked the lab called Electrophilic
Alkylation
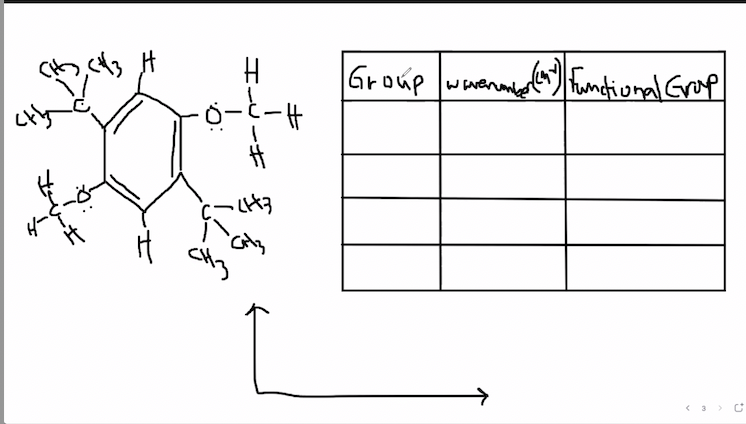
Transcribed Image Text:Group wavenm.e
undional Grap
< 3> C
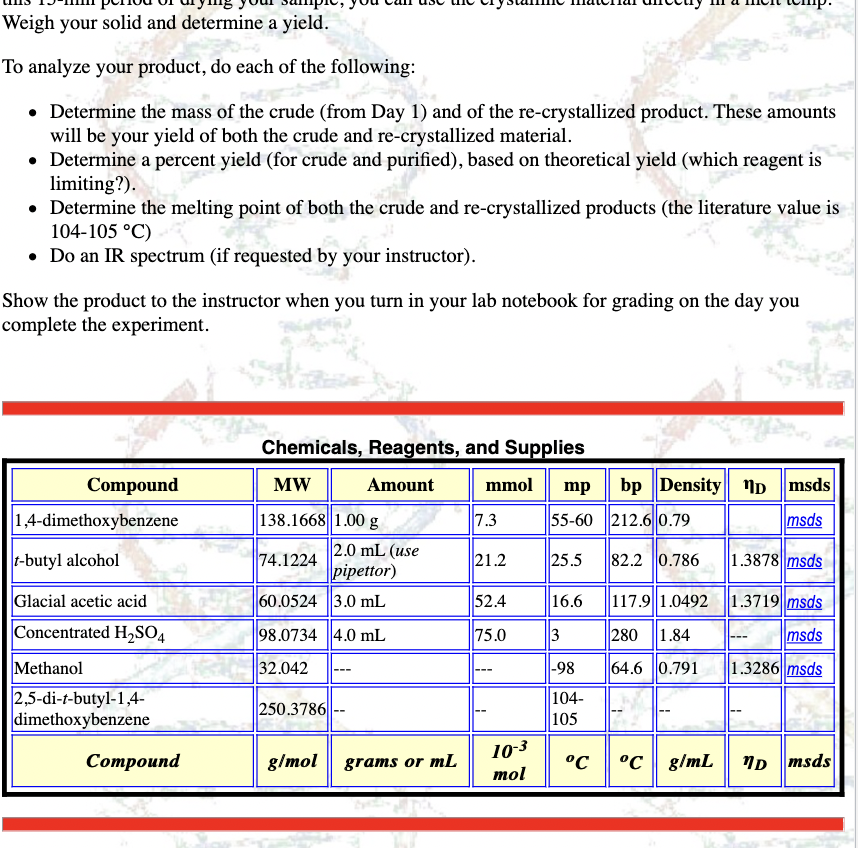
Transcribed Image Text:Weigh your solid and determine a yield.
To analyze your product, do each of the following:
• Determine the mass of the crude (from Day 1) and of the re-crystallized product. These amounts
will be your yield of both the crude and re-crystallized material.
• Determine a percent yield (for crude and purified), based on theoretical yield (which reagent is
limiting?).
• Determine the melting point of both the crude and re-crystallized products (the literature value is
104-105 °C)
• Do an IR spectrum (if requested by your instructor).
Show the product to the instructor when you turn in your lab notebook for grading on the day you
complete the experiment.
Chemicals, Reagents, and Supplies
Compound
MW
Amount
mmol
mp
bp Density Np msds
1,4-dimethoxybenzene
138.1668 1.00 g
7.3
55-60 212.6 0.79
msds
2.0 mL (use
pipettor)
60.0524 3.0 mL
t-butyl alcohol
74.1224
21.2
25.5
82.2 0.786
1.3878 msds
Glacial acetic acid
52.4
16.6
117.9 1.0492 1.3719| msds
Concentrated H,SO4
98.0734 4.0 mL
75.0
3
280 1.84
msds
---
Methanol
32.042
|-98
64.6 0.791
1.3286 msds
2,5-di-t-butyl-1,4-
dimethoxybenzene
104-
105
250.3786||
--
--
10-3
mol
°c °C g/mL
ND msds
Соmроund
g/mol
grams or mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
