H2(g) + Cl2(g) ----> 2 HClg) Use the following conversions: 1 mol = MW in g (2 d.p.) = 22.414 L How many grams of HCI (MW = 36.46 g HCI) will form from 42.5 L of Cl2 gas (MW = 70.90 g Cl2)?(Express as 'g HCI') 3._ 5. 1. 42.5 L C12 x- 2. 7. 4. 6. 1.--- 2.-- 3.-- 4. _--- 5.___
H2(g) + Cl2(g) ----> 2 HClg) Use the following conversions: 1 mol = MW in g (2 d.p.) = 22.414 L How many grams of HCI (MW = 36.46 g HCI) will form from 42.5 L of Cl2 gas (MW = 70.90 g Cl2)?(Express as 'g HCI') 3._ 5. 1. 42.5 L C12 x- 2. 7. 4. 6. 1.--- 2.-- 3.-- 4. _--- 5.___
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section: Chapter Questions
Problem 25PS: A gaseous organofluorine compound has a density of 0.355 g/L at 17 C and 189 mm Hg. What is the...
Related questions
Question
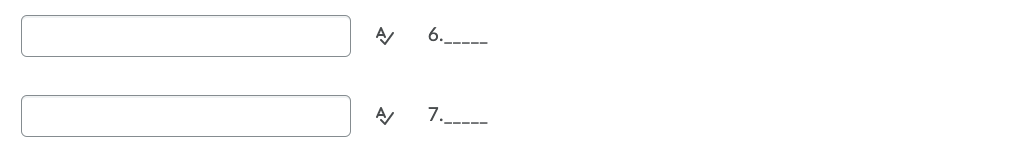
Transcribed Image Text:6.__-
7.---
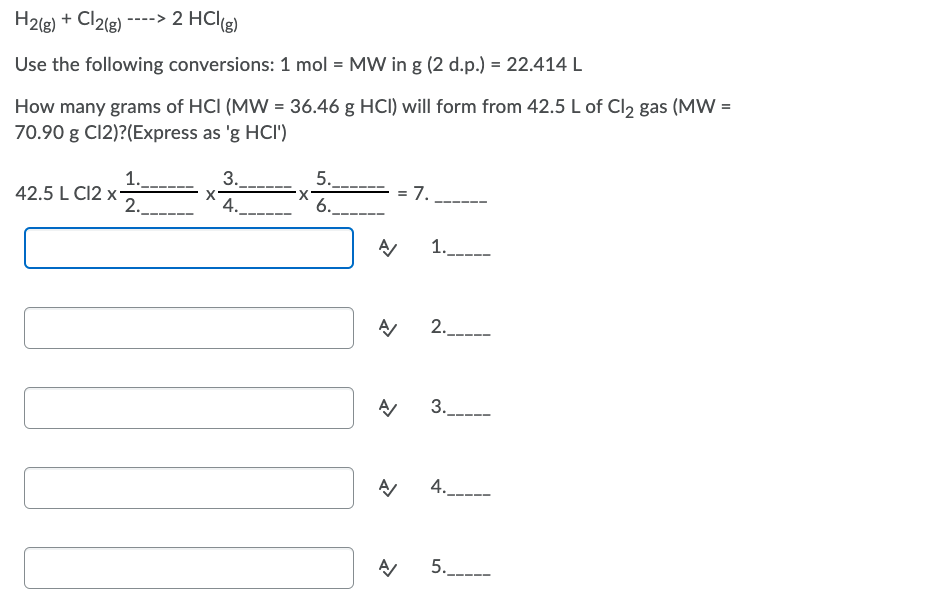
Transcribed Image Text:H2(g) + Cl2(g)
----> 2 HCl(g)
Use the following conversions: 1 mol = MW in g (2 d.p.) = 22.414 L
How many grams of HCI (MW = 36.46 g HCI) will form from 42.5 L of Cl2 gas (MW =
70.90 g C12)?(Express as 'g HCI')
3.
1.
42.5 L C12 x-
2.
5..
X.
6.
7.
X'
4.
1.---
2.---
3..
4.
5.----
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning