H2lg) reacts with N{g) to produce NH3(g). What mass of H2(g) is required toct completely and produce 4.80x100 molecules of t a. 0.797 mg b. 1.20 mg 2.41 mg d. 1.61 mg e. 1.07 mg
H2lg) reacts with N{g) to produce NH3(g). What mass of H2(g) is required toct completely and produce 4.80x100 molecules of t a. 0.797 mg b. 1.20 mg 2.41 mg d. 1.61 mg e. 1.07 mg
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 92QRT
Related questions
Question
Transcribed Image Text:Safari
File
Edit
View
History
Bookmarks
Window
Help
.learn squ.e
E-LEARNING SYSTEM (ACADEMIC)
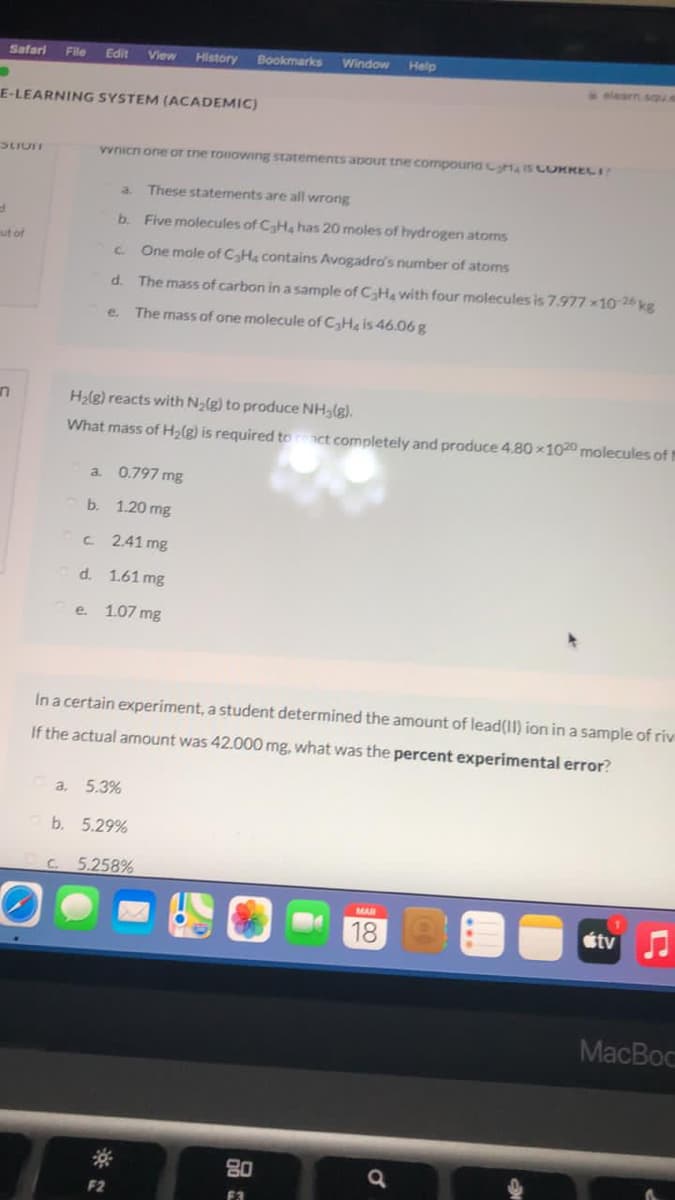
vvnicn one oT tne roiowing statements apout tne compouna IS CORKECI
a.
These statements are all wrong
b. Five molecules of CH, has 20 moles of hydrogen atoms
ut of
C.
One mole of CaHa contains Avogadro's number of atoms
d.
The mass of carbon in a sample of CHa with four molecules is 7.977 x10 26 kg
e.
The mass of one molecule of CHg is 46.06 g
H2lg) reacts with Na(g) to produce NH3(g).
What mass of H2(g) is required to
ct completely and produce 4.80 100 molecules of t
a. 0.797 mg
b. 1.20 mg
2.41 mg
d. 1.61 mg
e. 1.07 mg
In a certain experiment, a student determined the amount of lead(II) ion in a sample of riv
If the actual amount was 42.000 mg, what was the percent experimental error?
a,
5.3%
b. 5.29%
C.
5.258%
MAR
18
tv
MacBoc
80
F2
F3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
